
Am Fam Physician. 2025;112(1):20-21
Related Letter to the Editor: Surgery vs Corticosteroid Injections for Carpal Tunnel Syndrome
Author disclosure: No relevant financial relationships.

DETAILS FOR THIS REVIEW
Study Population: Adults 32 to 53 years of age; 84% women; varying severity of carpal tunnel syndrome; average duration of symptoms between 31 weeks and 3.5 years
Efficacy End Points: Primary outcomes: short-term (less than 3 months follow-up) and long-term (greater than 3 months follow-up) clinical improvement; secondary outcomes: symptoms, function, pain, and health-related quality of life; the Boston Carpal Tunnel Questionnaire was used in 8 of 14 studies to quantify symptoms and function
Harm End Points: Secondary outcomes: adverse effects (eg, painful neuroma, tender or hypertrophic scar, subluxation of flexor tendons, wound infection, complex regional pain syndrome, sectioning of motor branch of medial nerve) and need for surgery
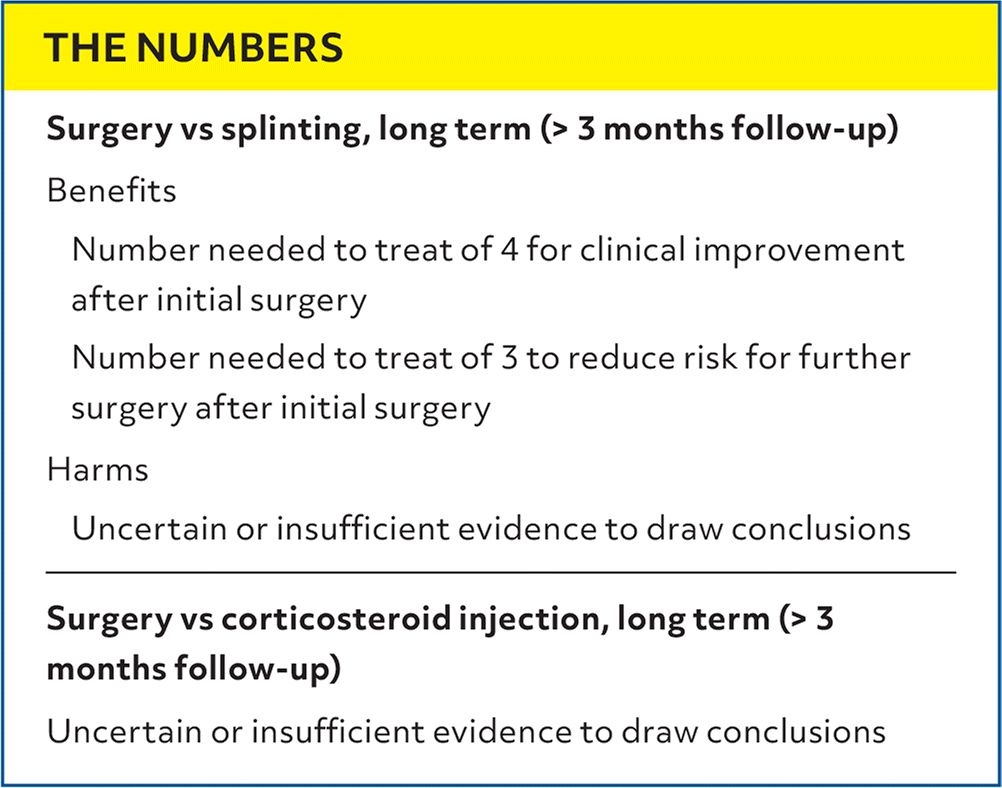
| Surgery vs splinting, long term (> 3 months follow-up) |
| Benefits |
| Number needed to treat of 4 for clinical improvement after initial surgery |
| Number needed to treat of 3 to reduce risk for further surgery after initial surgery |
| Harms |
| Uncertain or insufficient evidence to draw conclusions |
| Surgery vs corticosteroid injection, long term (> 3 months follow-up) |
| Uncertain or insufficient evidence to draw conclusions |
Subscribe
From $180- Immediate, unlimited access to all AFP content
- More than 125 CME credits/year
- Print delivery available
Issue Access
$59.95- Immediate, unlimited access to this issue's content
- CME credits
- Print delivery available
