
Am Fam Physician. 2025;112(2):117-118
Author Disclosure: No relevant financial relationships.
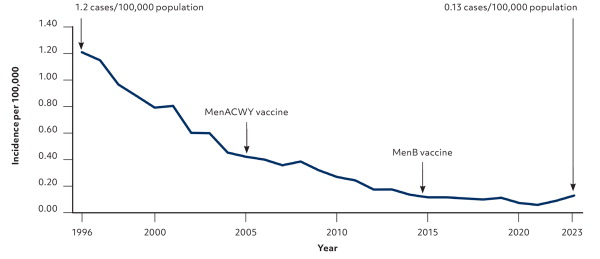
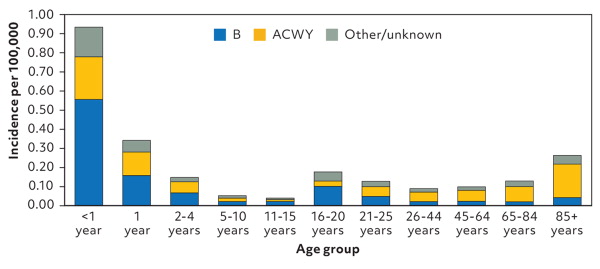
Invasive Neisseria meningitidis is a major cause of meningitis and septicemia and has five major serogroups (A, B, C, W, and Y). It is an uncommon illness that can lead to serious complications or death in previously healthy children and adolescents. The incidence of meningococcal disease in 2023 was 1.3 cases per 1 million people, with the highest risks in infants and children younger than 2 years, individuals ages 16 to 20 years, and adults older than 85 years (Figure 1 and Figure 2).1 This editorial discusses the use of meningococcal vaccines in adolescents and young adults at average risk of meningococcal infection.
Two quadrivalent meningococcal conjugate vaccines (MenACWY) and two serogroup B meningococcal vaccines (MenB) are recommended by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP). These vaccines are summarized in Table 1. The recommendations for use include one MenACWY dose at age 11 or 12 years and a booster at age 16 years. MenB is not recommended routinely but can be used after shared decision-making. Two doses are given at least 6 months apart at age 16 to 23 years (preferably age 16 to 18 years).2 The ACIP did not universally recommend MenB for older adolescents and young adults because of the very low incidence of disease, a short duration of protection, lack of prevention of carriage, and a high cost per benefit.
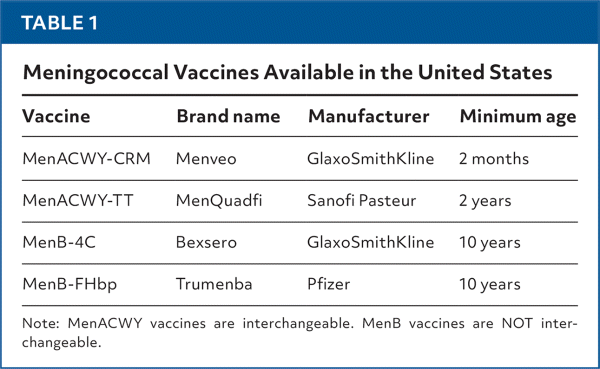
| Vaccine | Brand name | Manufacturer | Minimum age |
|---|---|---|---|
| MenACWY-CRM | Menveo | GlaxoSmithKline | 2 months |
| MenACWY-TT | MenQuadfi | Sanofi Pasteur | 2 years |
| MenB-4C | Bexsero | GlaxoSmithKline | 10 years |
| MenB-FHbp | Trumenba | Pfizer | 10 years |
Two pentavalent meningococcal vaccines (Penbraya, Penmenvy), containing all five serogroup antigens, are approved for use in the United States (Table 2). The ACIP began recommending Penbraya in 2023 and voted to include Penmenvy as another option at their meeting in April 2025. The pentavalent meningococcal vaccines may be administered in those 10 years and older, when MenACWY and MenB vaccines are indicated at the same visit. The ACIP does not recommend substituting a pentavalent vaccine for MenACWY or MenB when only one of those is indicated.3
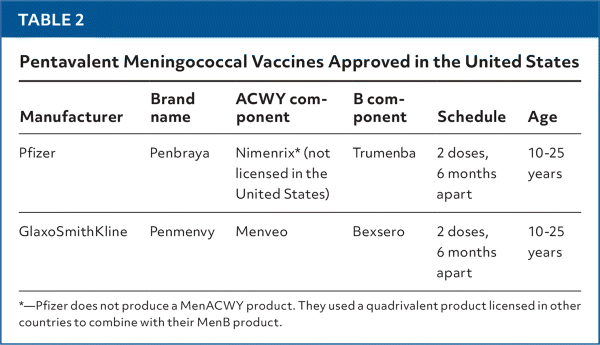
| Manufacturer | Brand name | ACWY component | B component | Schedule | Age |
|---|---|---|---|---|---|
| Pfizer | Penbraya | Nimenrix* (not licensed in the United States) | Trumenba | 2 doses, 6 months apart | 10–25 years |
| GlaxoSmithKline | Penmenvy | Menveo | Bexsero | 2 doses, 6 months apart | 10–25 years |
The MenACWY products are interchangeable. The use of one product is recommended, but not required, for both doses. However, it is important to note that the MenB products are not interchangeable. Administration of a B-component vaccine (monovalent or pentavalent) requires that subsequent B-component vaccine doses be from the same manufacturer.
Here is one common scenario: an 11-year-old patient receives a dose of MenACW Y, either quadrivalent product. At age 16, this adolescent receives a Men-ACWY booster as recommended and, after a discussion of the pros and cons, he also receives MenB. Two injections (MenACWY and MenB) or a single injection of a pentavalent vaccine are both acceptable options. If Penbraya is the pentavalent option used, the MenB second dose in 6 months should be Trumenba. If the pentavalent product is Penmenvy, the MenB second dose should be Bexsero.
Unfortunately, these combination vaccines, which were intended to simplify the vaccine schedule, have made it more complicated. The ACIP will be reconsidering adolescent meningococcal vaccine recommendations over the next year.
