
Am Fam Physician. 2025;112(3):244-246
Related article: Colorectal Cancer: Screening and Prevention
Author disclosure: No relevant financial relationships.
In the United States, the mortality rate of colorectal cancer (CRC) has decreased steadily over the past 40 years, but CRC remains the second overall leading cause of cancer deaths and disproportionately affects people with reduced access to health care.1 Regular periodic screening using US Preventive Services Task Force (USPSTF)-recommended strategies reduces CRC mortality risk.2
Noninvasive screening methods, such as stool-based testing, are more accessible and less expensive than invasive procedures; however, their effectiveness hinges on the patient receiving prompt follow-up colonoscopy after a positive result. Unfortunately, follow-up colonoscopy rates are low, and a Healthcare Effectiveness Data and Information Set screening measure for this procedure is not currently available to help guide improvements in follow-up colonoscopy rates.
Advantages of noninvasive stool-based tests (ie, high-sensitivity guaiac-based fecal occult blood testing [gFOBT], fecal immunochemical testing [FIT], and multitarget stool DNA or FIT-DNA testing) include screening without the need for an office visit, fewer harms compared with colonoscopy, and lower costs. A FIT-DNA testing strategy offers similar benefits, although with higher direct cost than with FIT testing.
Studies show similar CRC detection and mortality reduction rates between FIT and colonoscopy.3 A large randomized controlled trial found that invitation to FIT was noninferior to invitation to colonoscopy screening for CRC mortality after 10 years.4 Randomized controlled trials of gFOBT screening reported reductions of 13% to 33% in CRC mortality risk.5 Large FIT-based screening programs have reported reduced CRC incidence and mortality rates, as well as racial disparities in CRC incidence and mortality.6
FIT has wide patient acceptability and is the most widely recommended CRC screening test worldwide.7 A systematic review reported a 1.2-fold higher screening participation rate when FIT was offered rather than gFOBT.8 In one randomized controlled trial, the screening participation rate was 40.7% with FIT outreach vs 24.6% with colonoscopy outreach.9 FIT is convenient, requiring only a single sample that is typically collected in the comfort of home without requiring dietary or medication changes.
Stool-based screening tests offer the unique advantage of providing the ablity to complete screening through mailed outreach without requiring an office visit, which can be invaluable for people experiencing barriers to health care.10 Additionally, studies show FIT has higher sensitivity than gFOBT for detecting CRC and advanced precancerous lesions while maintaining comparable specificity.3 Among noninvasive tests, FIT offers the best balance of patient acceptability, cost, effectiveness, and need for colonoscopy resources. FIT with standardized quantitative autoanalysis offers advantages over point-of-care tests for screening delivery at scale.
The landscape of noninvasive CRC testing is rapidly expanding with a refined FIT-DNA test (Cologuard), a new multitarget stool RNA test (ColoSense), CRC-specific blood-based testing, and multicancer detection tests. An accurate blood-based test could expand screening options, but it requires an office visit. Currently available blood-based tests have low sensitivity for advanced precancerous lesions (13.2%) and stage 1 CRC (54.5%), and no direct evidence currently supports potential effects on health outcomes.11
For optimal CRC prevention and early detection, we recommend prioritizing noninvasive tests with sensitivity for advanced precancerous lesions of at least 30%.12 The USPSTF has not recommended any blood-based tests for screening, and they should not be used in place of evidence-based tests.
The advantages of noninvasive screening methods can be realized only when patients receive follow-up colonoscopy after positive test results, as emphasized by the USPSTF in its 2021 recommendation statement.2 In 2023, the Centers for Medicare and Medicaid Services classified follow-up colonoscopy for positive noninvasive test results as part of complete screening, eliminating coinsurance costs for the procedure. Unfortunately, in an Optum database study, only 43.3% of patients with a positive stool-based screening result underwent follow-up colonoscopy within 90 days.13 Potential reasons include failure to communicate results to patients, inappropriate retesting to confirm positive results, and social barriers.
Furthermore, the capacity to promptly perform colonoscopies after positive noninvasive screening test results is strained by overreliance on colonoscopy in primary care and gastrointestinal settings for primary screening and also screening at shorter intervals than recommended.14 The 10-year screening interval afforded by colonoscopy and misperceptions of this method's superiority may contribute to overreliance.
Noninvasive evidence-based tests and delivery strategies improve screening rates, but delays or failures in performing follow-up colonoscopy undermine the benefits. All patients with a positive noncolonoscopy test result should complete follow-up colonoscopy promptly, ideally within 3 to 6 months. Recommendations for practice are listed in Table 1.2,6,10,12
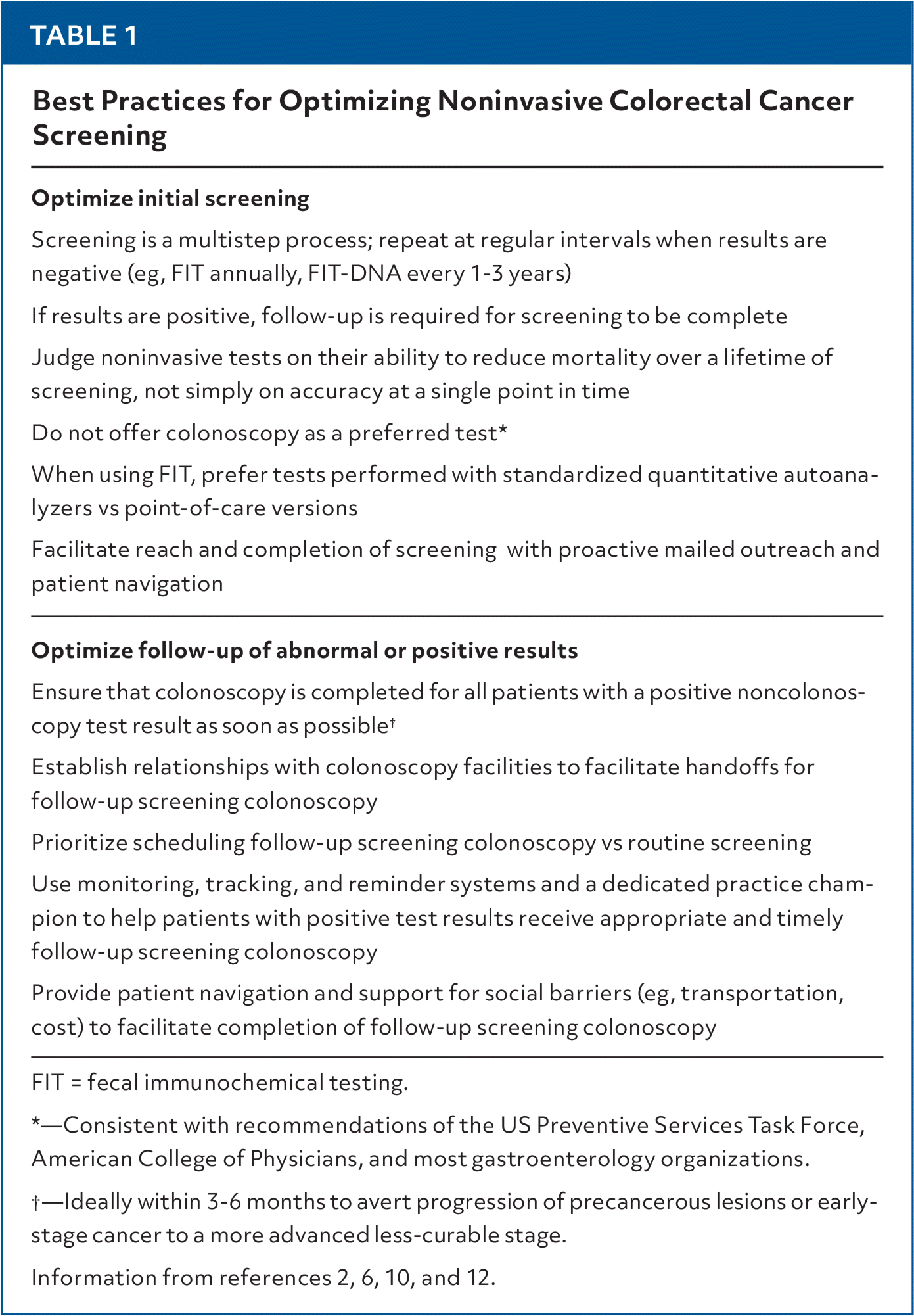
| Optimize initial screening |
| Screening is a multistep process; repeat at regular intervals when results are negative (eg, FIT annually, FIT-DNA every 1–3 years) |
| If results are positive, follow-up is required for screening to be complete |
| Judge noninvasive tests on their ability to reduce mortality over a lifetime of screening, not simply on accuracy at a single point in time |
| Do not offer colonoscopy as a preferred test* |
| When using FIT, prefer tests performed with standardized quantitative autoanalyzers vs point-of-care versions |
| Facilitate reach and completion of screening with proactive mailed outreach and patient navigation |
| Optimize follow-up of abnormal or positive results |
| Ensure that colonoscopy is completed for all patients with a positive noncolonoscopy test result as soon as possible† |
| Establish relationships with colonoscopy facilities to facilitate handoffs for follow-up screening colonoscopy |
| Prioritize scheduling follow-up screening colonoscopy vs routine screening |
| Use monitoring, tracking, and reminder systems and a dedicated practice champion to help patients with positive test results receive appropriate and timely follow-up screening colonoscopy |
| Provide patient navigation and support for social barriers (eg, transportation, cost) to facilitate completion of follow-up screening colonoscopy |
We call on the National Committee for Quality Assurance to add a national follow-up screening colonoscopy metric separately from the Healthcare Effectiveness Data and Information Set CRC screening measure to focus greater attention on attaining the benefits of noninvasive CRC screening. Furthermore, clinical delivery systems, the Centers for Medicare and Medicaid Services, and private payers should adopt and incentivize follow-up colonoscopy as a core clinical quality metric.
Clinicians should assure access to colonoscopy before initiating a noninvasive CRC screening program, and colonoscopy should not be offered as a preferred test. Rather, clinicians should engage patients in shared decision-making regarding available tests. Finally, we call on endoscopy services to prioritize scheduling colonoscopy for follow-up of positive noninvasive screening results over routine screening use.
