COVID-19 diagnosis coding explained in a flowchart
Diagnosis coding for COVID-19 is complicated, in part because the World Health Organization (WHO) created two diagnosis codes for COVID-19, but so far the United States has adopted only one. The WHO’s codes are as follows:
- U07.1 – COVID-19, virus identified (lab confirmed),
- U07.2 – COVID-19, virus not identified (clinically diagnosed).
The WHO’s intent was for U07.1 to be assigned to cases with a lab-confirmed diagnosis and for U07.2 to be used for cases with a clinical diagnosis. The United States adopted U07.1 as an immediate, off-cycle update to the ICD-10 code set but did not adopt U07.2 because it was released later. Therefore, in the United States, the words “virus identified” are left out of the description for U07.1, which leaves room for interpretation about when to use the code.
The predicament
Without having a diagnosis code specifically for clinically diagnosed (rather than lab-confirmed) COVID-19, U.S. physicians are left without clear guidance.
How do we properly document and code for a patient who is clinically diagnosed? If we only use symptom codes, patients will most likely not be covered by their payers for COVID-19 care and they also will not be included in a disease registry. This is important because many payers are waiving any cost-share responsibility for patients diagnosed with COVID-19. However, in order for the claim to be accepted as a COVID-19 related service, it must have a COVID-19 related diagnosis code. Also, if the patient is in a disease registry, it allows for adequate follow-up, especially if we start to use serologic testing for patients. Sensitivity to the COVID-19 reverse transcriptase (RT-PCR), or diagnostic, tests has been reported to be 70% with a single respiratory swab.1 Consequently, in some instances, patients clinically appear to have COVID-19, but their RT-PCR is not positive until subsequent tests. These patients should be diagnosed with COVID-19, even though the initial labs were negative.
Then there is the question of using serologic (antibody) testing for diagnosing COVID-19. At the time the ICD-10 code was introduced, only RT-PCR testing was available. Now, physicians have more access to serologic tests; however, there is the issue of how to interpret these tests and their use in diagnosing patients with COVID-19. According to the Centers for Disease Control and Prevention (CDC), serologic testing can be offered to support a diagnosis of COVID-19 for patients who present late. Patients presenting 9 to 14 days after illness onset can be tested with the antibody test, in addition to the RT-PCR test to maximize sensitivity.2 A positive serologic test result indicates past or present COVID-19 infection. But it could be a false positive; therefore, serologic testing should not be the only factor in diagnosing COVID-19.3
If a patient has clinical symptoms consistent with COVID-19 and/or has had exposure, and the patient has a negative COVID-19 RT-PCR test, how should the serologic test be interpreted? If the serologic test reveals IgM (-) and IgG (+), should the patient be diagnosed with COVID-19? If the patient has no symptoms or no known exposure, a negative COVID-19 RT-PCR test, and the serologic test reveals IgM (-) and IgG (+), should this patient be diagnosed with COVID-19? Or is there the need for a new ICD-10 code indicating previous infection or previous exposure identified by serology?
The recommendation
Without having a diagnosis code for both lab-confirmed COVID-19 and clinically diagnosed COVID-19, we only have one option: U07.1 – COVID-19. For purposes of vital statistics reporting, the CDC’s National Center for Health Statistics (NCHS) has confirmed that U07.1 can be used for both lab-confirmed and clinically diagnosed patients who have died.4 But the CDC’s broader guidance on coding for living patients again leaves room for interpretation. CDC has stated, “Code only a confirmed diagnosis of the 2019 novel coronavirus disease (COVID-19) as documented by the provider, documentation of a positive COVID-19 test result, or a presumptive positive COVID-19 test result. In this context, ‘confirmation’ does not require documentation of the type of test performed; the provider’s documentation that the individual has COVID-19 is sufficient.”5 The phrase “as documented by the provider” can be interpreted to mean as clinically diagnosed by the provider.
Different diagnosis algorithms can be used for diagnosing patients with COVID-19. Physicians should take prevalence and incidence in their own community into consideration when assigning a clinical diagnosis, especially in the absence of a positive test.
A flowchart
Here is a four-part flowchart that our organization developed to help physicians navigate these waters and properly assign diagnosis codes related to COVID-19 encounters. No one algorithm will fit every health care system. It may need to be tailored to your geographic location since prevalence and incidence vary greatly not only throughout the United States but even throughout states, where pockets of COVID-19 surges are emerging.
Click on each image below to enlarge, print, or download. Or download the pdf version.
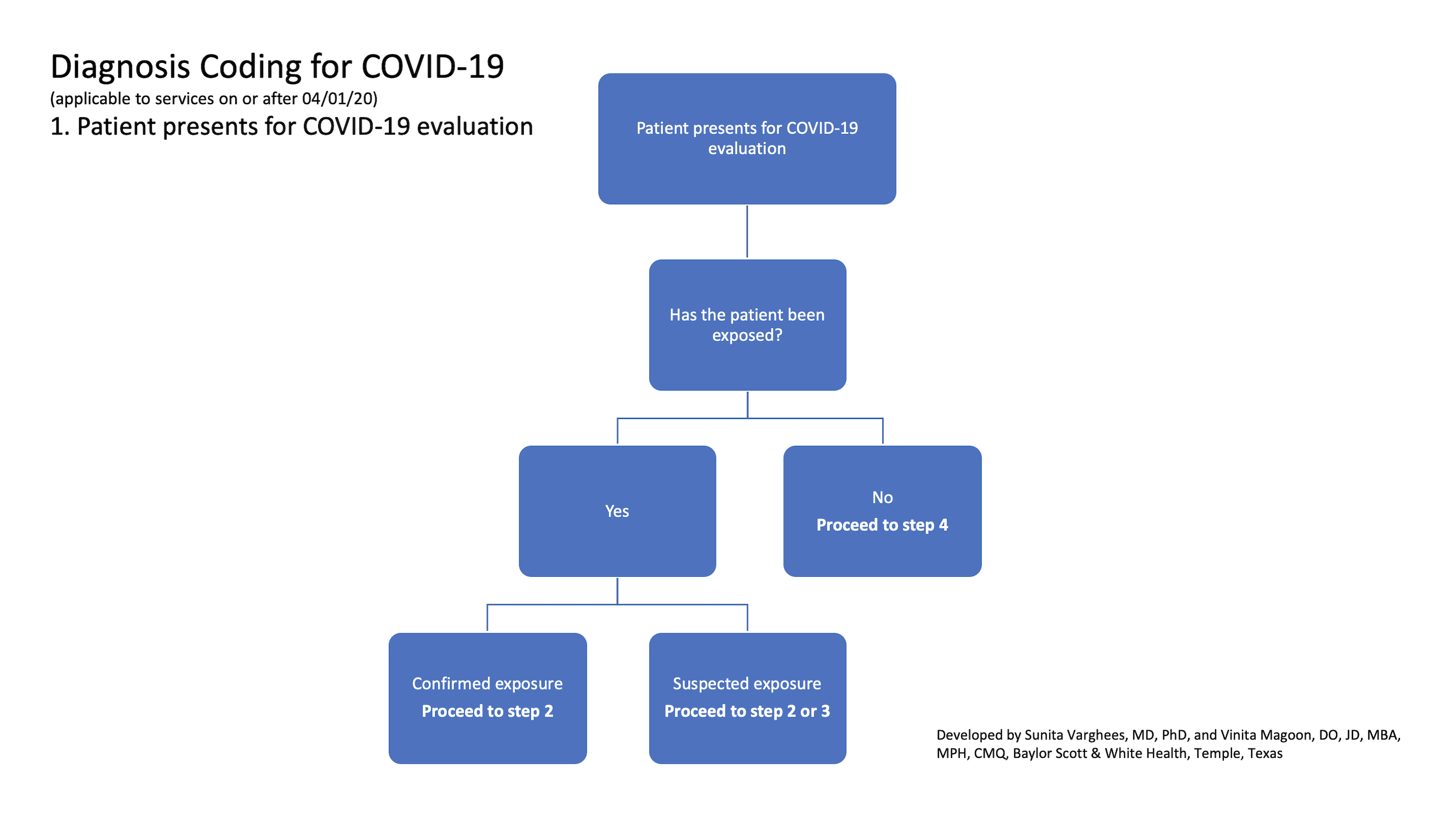
1. Patient presents for COVID-19 evaluation
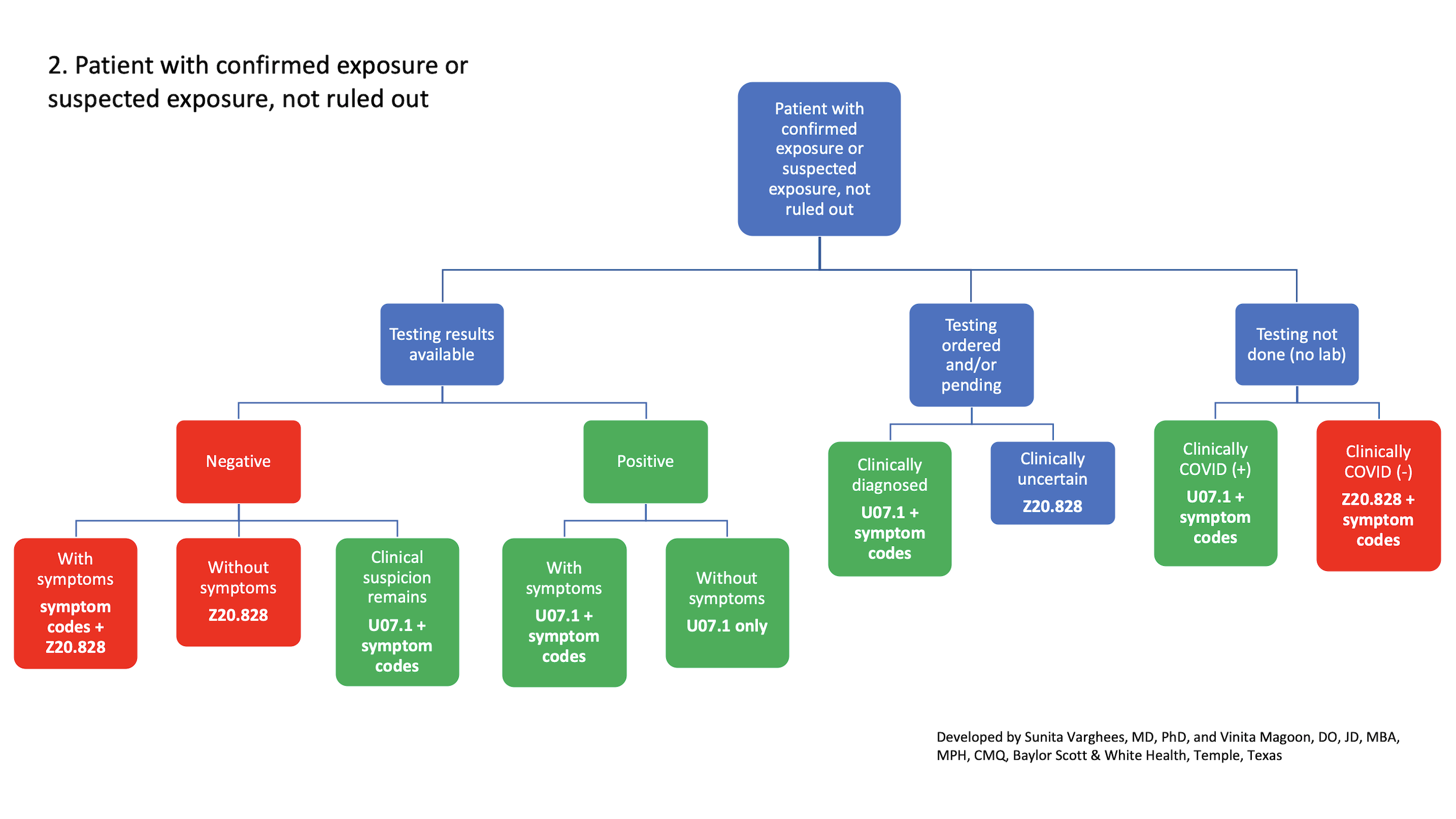
2. Patient with confirmed exposure or suspected exposure, not ruled out
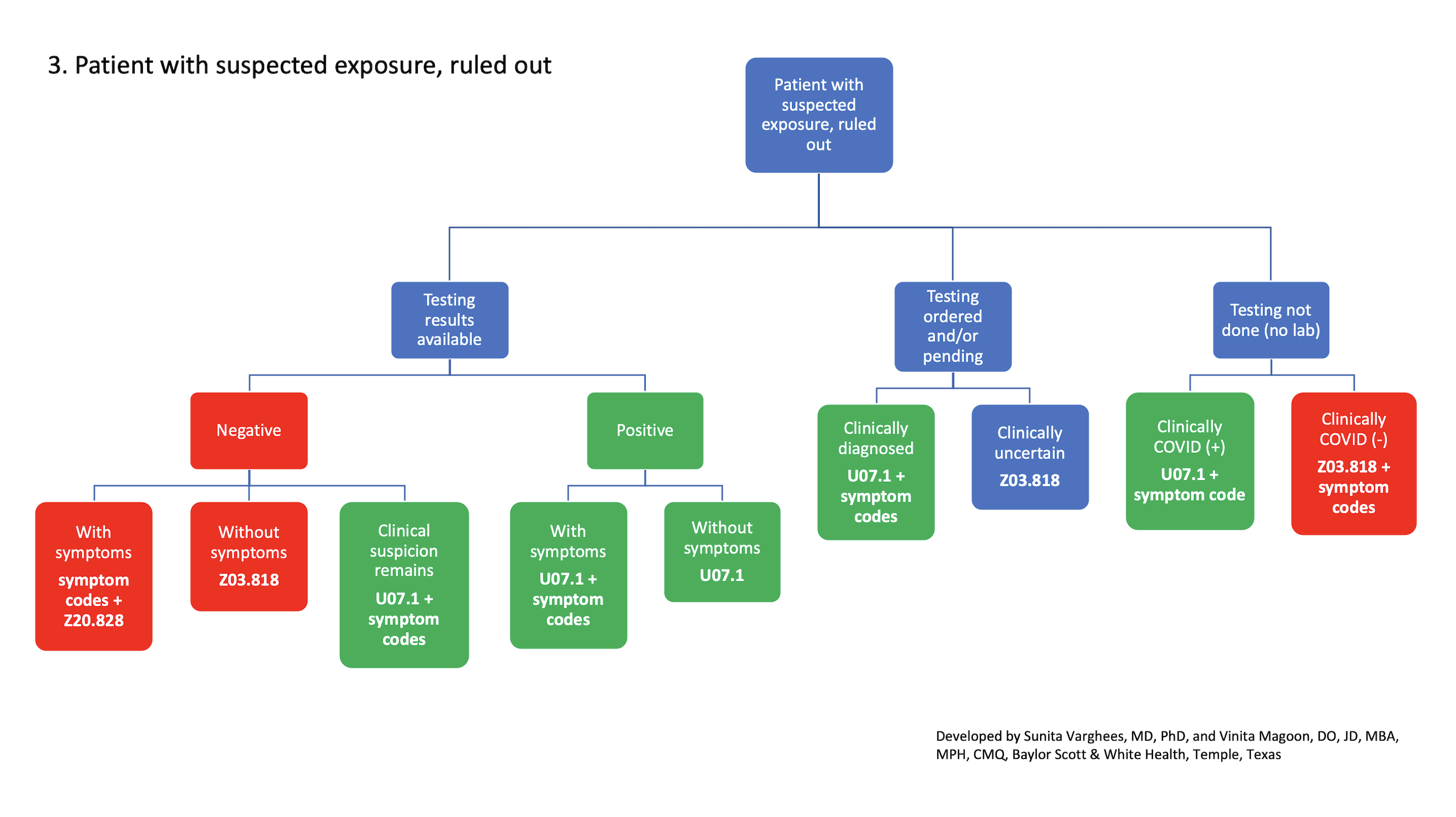
3. Patient with suspected exposure, ruled out
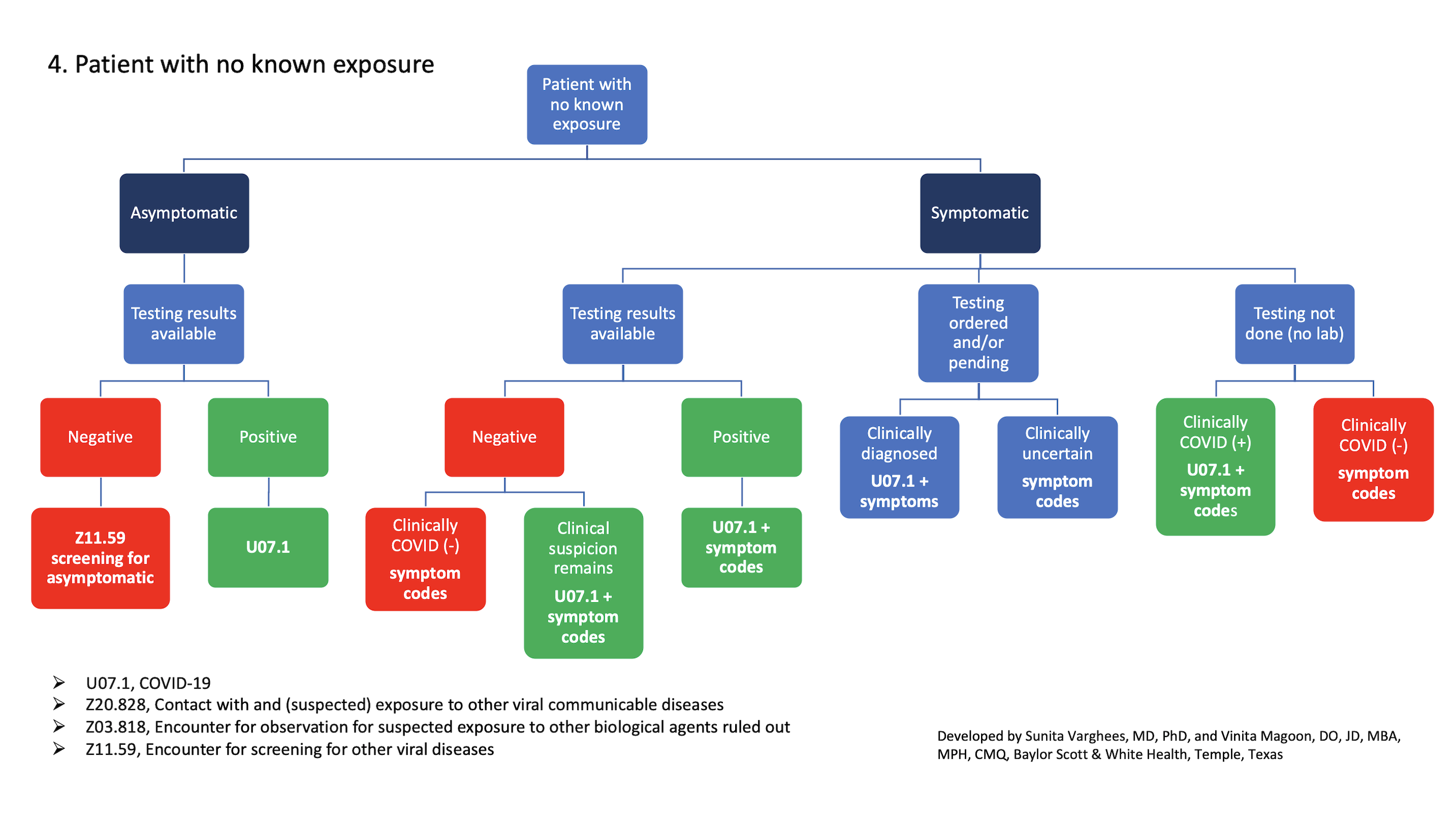
4. Patient with no known exposure
— Sunita Varghees, MD, PhD, CMQ, and Vinita Magoon, DO, JD, MBA, MPH, CMQ, of Baylor Scott & White Health, Temple, Texas
1. Yicheng Fang, Huangqi Zhang, Jicheng Xie, et al. Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology. Feb. 19, 2020. doi: 10.1148/radiol.2020200432
2. Interim guidelines for COVID-19 antibody testing. Centers for Disease Control and Prevention. Reviewed May 23, 2020. Accessed June 23, 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html
3. Public health considerations: serologic testing for COVID-19. Association of Public Health Laboratories. May 7, 2020. Accessed June 23, 2020. https://www.aphl.org/programs/preparedness/crisis-management/documents/serologic-Testing-for-COVID-19.pdf
4. Guidance for certifying deaths due to coronavirus disease 2019 (COVID-19). Centers for Disease Control and Prevention National Center for Health Statistics. April 2020. Accessed June 23, 2020. https://www.cdc.gov/nchs/data/nvss/vsrg/vsrg03-508.pdf
5. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. Centers for Disease Control and Prevention National Center for Health Statistics. Accessed June 23, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf
Get "Quick Tips" in your inbox
Sign up to receive FPM's free, weekly e-newsletter, "Quick Tips & Insights," featuring practical, peer-reviewed advice for improving practice, enhancing the patient experience, and developing a rewarding career.
Other Blogs
- Getting Paid from FPM journal
- AFP Community Blog
- AAFP Voices Blog
Disclaimer: The opinions and views expressed here are those of the authors and do not necessarily represent or reflect the opinions and views of the American Academy of Family Physicians. This blog is not intended to provide medical, financial, or legal advice. All comments are moderated and will be removed if they violate our Terms of Use.