
When screening decisions aren't clear, this process can help you help your patients.
Fam Pract Manag. 2017;24(3):5-10
Author disclosures: no relevant financial affiliations disclosed.

Consider the following patient scenarios:
1. Nancy, a longtime patient of yours, comes to your clinic for a physical. While you are catching up with her, she tells you that the mom of one of her son's classmates was just diagnosed with breast cancer at the age of 41. Nancy is 45 years old and is now concerned that she has never had a mammogram.
2. Shari is a 65-year-old Caucasian woman with no chronic medical problems. You don't see her very often because she doesn't like to visit the doctor, but she's here for a physical. She is a current smoker with a 50 pack-year history of smoking. You see a note from your nurse saying that Shari would qualify for lung cancer screening.
3. Bob is a 58-year-old African American man who presents for a physical with no complaints. He has well-controlled hypertension and hyperlipidemia. He had a prostate-specific antigen (PSA) test in the past that was in the normal range, and his family history is negative for prostate cancer. His wife suggested he should get screened again based on an article she read.
In cancer screening discussions such as these, where patients may have more than one medically reasonable option, shared decision making can be a useful tool for helping patients decide what to do. This article presents “Three models of shared decision-making” and “Three case studies” showing how to use the models in a busy primary care practice.
What is shared decision making, and why do it?
Shared decision making is simply a process that aids a physician and patient in selecting the optimal test or treatment for the patient. It involves a bidirectional flow of information. (See “Shared decision making vs. usual care.”) The physician provides information about the disease, the screening service, and risks and benefits; the patient provides his or her thoughts and values; and together they make a decision. Shared decision making is distinct from informed decision making, where the physician provides information to the patient and then the patient makes the decision.
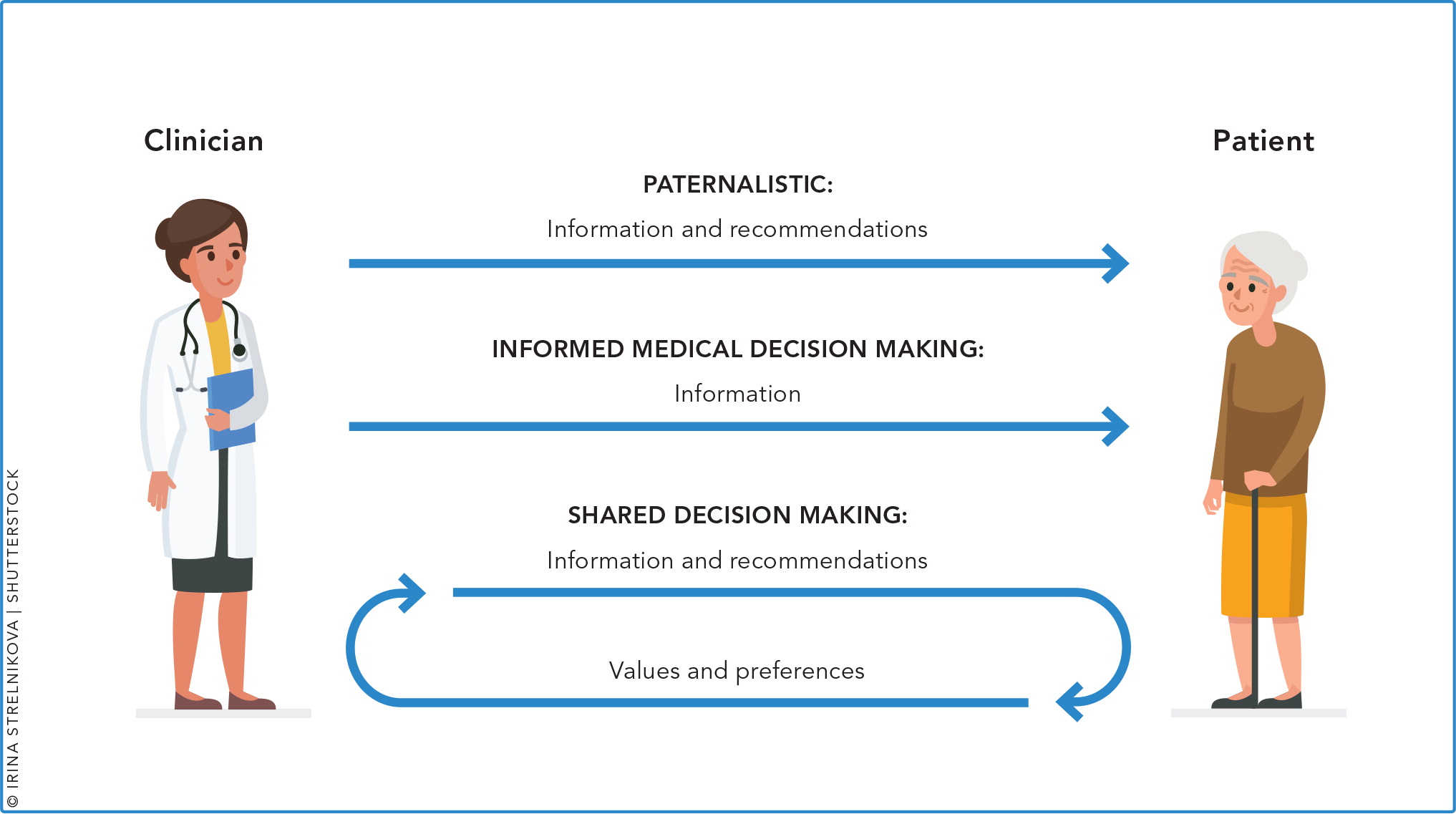
Although shared decision making is not appropriate in clinical scenarios where the medical treatment is clear, such as antibiotics for meningitis or anticoagulation for a pulmonary embolus, it proves beneficial in situations where more than one treatment or screening decision is valid. With cancer screening, there are many options for primary care patients and good evidence that early detection can lead to decreased mortality and morbidity. But most of the screening methods also have possible harmful effects including over-diagnosis or over-treatment, anxiety related to false positive results, and discomfort or harmful effects from diagnostic procedures. Patients should understand all of this information and consider their personal needs and values in order to make a wise decision about screening.
Shared decision making provides a patient-centered approach to care, which the Institute of Medicine recommended in its 2001 Crossing the Quality Chasm report.1 In fact, some have described shared decision making as “the pinnacle” of patient-centered care.2 Shared decision making can improve patient satisfaction and communication, potentially reducing medical malpractice claims.3,4,5 There is also some evidence that the use of shared decision making can reduce health inequalities among underserved populations.6 Additionally, when shared decision-making conversations include decision aids, they can improve care. A recent Cochrane review found that, compared with regular care, use of decision aids had the following results:7
Increased patients' knowledge,
Increased the proportion of people who had an accurate risk perception of the disease,
Increased the proportion of people who chose an option that was in line with their values,
Decreased decisional conflict,
Had a positive effect on clinician-patient communication,
Had a variable effect on length of visit (from −8 minutes to +23 minutes, with a median increase of 2.5 minutes per visit).
Shared decision making is an essential part of some clinical recommendations. For example, in its 2016 recommendations for breast cancer screening, the U.S. Preventive Services Task Force (USPSTF) gave a “C” recommendation for mammography in women ages 40 to 49 and recommended individual decision making based on values and personal risk.8 In addition, to get Medicare to pay for a computed tomography (CT) for lung cancer screening, physicians need to document that they had a shared decision-making conversation with the patient and talked about the risks and benefits of screening.
Even with all the evidence documenting that shared decision making improves patient care, it is not used frequently in primary care practices. Often, physicians feel that they do not have enough time during the visit or that shared decision making is not applicable to their patient or the clinical situation.9 Understanding the benefits of shared decision making and simple ways to do it can increase adoption.
VIDEO: SHARED DECISION MAKING IN BREAST CANCER SCREENING
Sarina Schrager, MD, demonstrates shared decision making with a patient considering mammography.
Six steps to shared decision making
The Informed Medical Decisions Foundation, now Healthwise, has identified six key steps that all shared decision-making conversations should include:10
Invite the patient to participate. This key first step informs patients that they have options in cancer screening and their values and preferences are an important part of the decision whether to get a particular screening test.
Present the options. For example, there are multitudes of ways to screen for colon cancer. For breast cancer screening, women 40 years and older can choose to get a mammogram or not.
Provide information on benefits and risks. A man considering a PSA test needs to know what a positive result means, what the risks of prostate biopsy are, and the effects of overdiagnosis (i.e., detection of low-grade cancer that would never have affected his life). He should also know that catching an aggressive cancer early may save his life.
Assist patients in evaluating options based on their goals and concerns. For example, if an elderly man does not want to have surgery in any situation because of a bad reaction he had in the past, then maybe lung cancer screening is not a good choice for him.
Facilitate deliberation and decision making. The primary care physician can help patients make decisions based on their ongoing relationship and experiences treating other illnesses. Cancer screening decisions do not need to be made urgently but can be discussed during a series of visits.
Assist patients in following through on their screening decisions. Members of the primary care team can aid patients in setting up appointments, provide information about tests, and help address any barriers to getting the desired screening.
Numerous models of shared decision making are available to primary care physicians to use in cancer screening. All of the models incorporate the six key areas of shared decision making described above. We have summarized three models that we think are the most useful in cancer screening: the Agency for Healthcare Research and Quality's SHARE method, the 5 As method described by the USPSTF, and the “IAIS” model described in a paper by David Price, MD.4,11,12
THREE MODELS OF SHARED DECISION MAKING
Of the numerous models of shared decision making available to primary care physicians, three are most useful in cancer screening.
SHARE model:
|
5 As model:
|
IAIS model:
|
Resources to help with common challenges
For a majority of primary care clinicians, the most difficult part of shared decision making is assessing the patient's values. It involves asking patients, “What matters to you?” and then figuring out how to integrate the answer into clinical decision making. One of the most commonly used values clarification tools is the Ottawa Personal Decision Guide. This guide is available for free online and provides patients with a structured method for reviewing treatment or screening options and weighing the importance of each option based on their values. The Ottawa tool does not focus on any condition specifically but instead offers a framework for considering the harms and benefits of treatment or screening decisions.
Another challenge for physicians is figuring out how to present information about screening in ways patients can understand. Ideally, physicians would have a conversation with their patients that informs them of the evidence behind a specific cancer screening test, the benefits (lives saved, cancers found early, etc.), and the possible harms (false positives, anxiety about further testing, unnecessary biopsies, overdiagnosis, etc.) and then help them make a decision. Unfortunately, much of that information is complex, and many guidelines either do not quantify harms and benefits or present them in an uneven manner (e.g., noting harms but not benefits, or vice versa).13
Sharing information about risk can be complex. Many clinicians tend to use language that is technical. For example, they might say, “This test has a 3 percent false positive rate,” or “The specificity of the test is 97 percent.” A better approach would be to convert this information into absolute risk and say, “If 100 people have this test, three will have a positive result even though they don't have cancer.”
In fact, a recent systematic review found that absolute risk formats as well as visual aids can help patients understand more complicated topics.14,15 Visualizing Health, a joint project of the Robert Wood Johnson Foundation and the University of Michigan Center for Health Communications Research, offers colorful graphs and pictures to help physicians communicate information about risk. In addition, visual aids are often found in decision aids.
Decision aids are helpful to convey complex information about a specific topic. The Ottawa Personal decision aid is an example of an aid that can be printed out and given to patients. Online resources for decision aids are available as well. (See “Online decision aid resources.”)
ONLINE DECISION AID RESOURCES
HealthDecision (http://www.healthdecision.org) offers interactive, individualized decision aids for breast and lung cancer screening. These decision aids are designed to be used jointly by the clinician and patient and can be integrated into the electronic health record.
Healthwise (http://www.healthwise.org/products/decisionaids.aspx) provides a variety of decision aids for patients to use themselves.
Agency for Healthcare Research and Quality (http://bit.ly/2ovA4OJ) provides a decision aid for lung cancer screening designed for patients to use themselves.
Option Grid (http://optiongrid.org/), from the Dartmouth Institute for Health Policy and Clinical Practice, provides numerous decision grids that help patients with screening and treatment decisions.
The Ottawa Patient Decision Aids Inventory (https://decisionaid.ohri.ca/azinvent.php) provides a clearinghouse of decision aids on a variety of topics.
Getting started
All primary care clinicians have the capacity to use shared decision making in clinical encounters. It provides a useful structure for discussing cancer screening options. To get started, choose the model that you are most comfortable with and that works well for your practice. Then, begin with just one patient who is facing a screening decision and might benefit from a conversation. Build on what you learn from that experience, and gradually expand your use of shared decision making. Your patients will appreciate and benefit from your efforts to take a more patient-centered approach to their care.
THREE CASE STUDIES
CASE STUDY 1: USING THE 5 As MODEL TO TALK ABOUT BREAST CANCER SCREENING.
Nancy, a longtime patient of yours, comes to your clinic for a physical. While you are catching up with her, she tells you that the mom of one of her son's classmates was just diagnosed with breast cancer at the age of 41. Nancy is 45 years old and is now concerned that she has never had a mammogram.
You first assess Nancy's risk of breast cancer. After turning your computer screen so that Nancy can see, you use the HealthDecision website as an interactive tool to determine Nancy's individual risk of developing breast cancer. The program suggests Nancy is at average risk, and you explain that out of 100 people just like her, one to two people would develop breast cancer in the next 10 years.
You advise Nancy on the current recommendations, benefits, harms, alternatives, and uncertainties of mammography screening for a woman with her characteristics. You continue using the HealthDecision website to visually demonstrate statistics about mammography screening. Following USPSTF recommendations, you tell Nancy that you would recommend she begin screening at age 50. However, you also acknowledge the ongoing debate about whether women at average risk for breast cancer should begin screening in their 40s and obtain a baseline mammogram. You ask Nancy to reflect on the harms and benefits of starting mammography screening, eliciting her values, concerns, and preferences. Nancy knows that going in for extra images or a biopsy would make her very nervous; however, she thinks waiting until she is age 50 to get her first mammogram would give her even more anxiety. Nancy explains that she interacts often with the woman recently diagnosed with breast cancer at her son's school and doesn't think she'd be able to see her go through treatment without being reassured that she herself was cancer-free.
You and Nancy agree on a breast cancer screening plan. Nancy would like a mammogram in the near future. If the mammogram is normal, she feels comfortable waiting until age 50 for her next mammogram.
With this plan in place, you direct your staff to assist Nancy in scheduling a mammogram for next month and arrange a follow-up appointment to discuss her results and revisit her screening plan. Her breast density on mammography may affect her overall risk of breast cancer and thereby affect her future screening plans.
CASE STUDY 2: USING THE SHARE METHOD TO TALK ABOUT LUNG CANCER SCREENING.
Shari is a 65-year-old Caucasian woman with no chronic medical problems. You don't see her very often because she doesn't like to visit the doctor, but she's here for a physical. She is a current smoker with a 50 pack-year history of smoking. You see a note from your nurse saying that Shari would qualify for lung cancer screening.
After a few minutes of greetings and catching up, you seek participation, asking Shari if she would be willing to talk about lung cancer screening. She agrees to talk about it, and you help her explore and compare options by going over the process of a lung CT, the sensitivity and specificity, what happens if the radiologist finds something, the risks from a biopsy, the possibility of needing frequent follow up CTs if the radiologist finds a nodule, etc. You also bring up smoking cessation, but she is not ready to talk about it yet.
Next you assess her values and preferences. Shari talks about not wanting frequent contact with the health profession and desires to live out her life without worrying about cancer. She remembers watching her mother die from lung cancer, which was horrible. Her mother never quit smoking and waited to go to the doctor until she could barely breathe. After a few more minutes of discussion about the benefits and risks of screening, you reach a decision with your patient. Shari decides to go ahead with a lung CT. She also agrees to think about quitting smoking.
Together, you evaluate her decision and agree that for her the benefits of screening outweigh the risks. You schedule a follow-up visit in two months to talk more about smoking cessation.
CASE STUDY 3: USING THE IAIS MODEL TO TALK ABOUT PROSTATE CANCER SCREENING.
Bob is a 58-year-old African American man who presents for a physical with no complaints. He has well-controlled hypertension and hyperlipidemia. He had a PSA test in the past that was in the normal range, and his family history is negative for prostate cancer. His wife suggested he should get screened again based on an article she read.
You invite his perspective and concerns, asking him what he thinks about it. He says he doesn't know much about prostate cancer, but his friend had surgery on his prostate and is now incontinent. He says he would do anything to avoid having a catheter.
You acknowledge his concerns about possible complications from prostate surgery. You then instruct him about his increased risk of prostate cancer due to being African American. You talk about the accuracy of the PSA test and what a biopsy would be like if the PSA were elevated. You then talk about the fact that detecting prostate cancer early can save lives, but doctors never know which cancers are going to be aggressive so we end up treating everyone similarly. You talk about the risks from prostatectomy including incontinence and erectile dysfunction. You also talk to him about the different recommendations regarding routine screening for prostate cancer (USPSTF was against it, but draft recommendations would allow for selective screening; the American Academy of Family Physicians and American College of Preventive Medicine are against it; and the American College of Physicians and American Urological Association recommend that the decision be based on shared decision making).
His initial decision is to not get a PSA test, but he wants to read more about it and talk to his wife. You summarize your discussion in writing for him to take home.
