
A more recent article on pharmacologic treatment of depression is available.
Am Fam Physician. 2015;92(2):94-100
Patient information: See related handout on treating depression with medicine, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
One in 11 U.S. adults currently meets diagnostic criteria for major depressive disorder, and a similar number report that they have taken an antidepressant medication in the past 30 days. In the primary care population, medications are modestly superior to placebo in achieving remission, with a number needed to treat of seven or eight for selective serotonin reuptake inhibitors and seven to 16 for tricyclic antidepressants. The benefit of antidepressants over placebo is more pronounced in patients with severe depression. Second-generation antidepressants are generally considered first-line therapy. Specific therapy choice should be based on cost, patient preference, and adverse effect profile. About two-thirds of patients receiving second-generation antidepressants experience at least one adverse effect during treatment. Nausea and vomiting are the most common reasons for discontinuation of therapy. The optimal treatment duration is unclear, but clinical guidelines suggest four to 12 months for an initial episode of major depression. Patients with recurrent depression may benefit from prolonged treatment. High-quality evidence is lacking on the benefits and harms of antidepressant use in pregnancy. It is unclear whether selective serotonin reuptake inhibitor use in breastfeeding mothers causes adverse effects in their infants, but sertraline and paroxetine transfer to breast milk in lower concentrations than other antidepressants. Consensus guidelines recommend a “start low, go slow” approach to antidepressant therapy in older persons; preferred medications include citalopram, escitalopram, sertraline, mirtazapine, and venlafaxine.
Depression is a common disease, with a 12-month prevalence of 18% in the primary care population.1 A national survey conducted from 2006 to 2008 showed that 9% of U.S. adults met the criteria for depression in the two weeks before the survey.2 From 2007 to 2010, antidepressants were the third most common class of prescription drug taken by Americans of all ages, with 8.7% having taken them in the past 30 days; this compares with 1.8% in 1988 to 1994.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Selective serotonin reuptake inhibitors are more likely than placebo to produce depression remission in the primary care population. | B | 1 |
| Serotonin-norepinephrine reuptake inhibitors are slightly more likely than selective serotonin reuptake inhibitors to improve depression symptoms, but they are associated with higher rates of adverse effects such as nausea and vomiting. | B | 6, 41 |
| For treatment-naive patients, all second-generation antidepressants are equally effective. Medication choice should be based on patient preferences, with adverse effect profiles, cost, and dosing frequency taken into consideration. | C | 42 |
| Antidepressants are most effective in patients with severe depression. | A | 44–46 |
| Preferred agents for older patients with depression include citalopram (Celexa), escitalopram (Lexapro), sertraline (Zoloft), mirtazapine (Remeron), venlafaxine, and bupropion (Wellbutrin). Because of higher rates of adverse effects in older adults, paroxetine (Paxil) and fluoxetine (Prozac) should generally be avoided. | C | 50 |
| Treatment for a first episode of major depression should last at least four months. Patients with recurrent depression may benefit from prolonged treatment. | C | 42 |
Are Antidepressants Effective?
Antidepressant medication is modestly superior to placebo for treatment of major depressive disorder in the primary care population.
EVIDENCE SUMMARY
Although the use of antidepressants is widespread, research has shown mixed effectiveness. Selective publication of positive results (publication bias) causes meta-analyses of published trials to exaggerate the benefits of treatment.4 A review of published clinical trials of 12 antidepressants approved by the U.S. Food and Drug Administration (FDA) between 1987 and 2004 suggested that 94% of trials had positive results; in contrast, the FDA analysis of all studies showed that only 51% had positive results.4
A Cochrane meta-analysis of antidepressant use in the primary care population concluded that antidepressants are modestly better than placebo for the treatment of major depressive disorder.1 All studies of selective serotonin reuptake inhibitors (SSRIs) were of short duration and were industry funded. The number needed to treat ranged from seven to 16 for tricyclic antidepressants (TCAs) and seven or eight for SSRIs. Numbers needed to treat were generated from studies with dichotomous remission outcomes: either 50% reduction from the initial score, or a score of less than 8 on the Hamilton Rating Scale for Depression. In older studies, TCAs were shown to be only minimally superior to active placebos (i.e., those with adverse effects similar to those of antidepressants).5 It is unclear how active placebos might compare with SSRIs.
What Are the Harms of Treatment?
About 63% of patients receiving second-generation antidepressants (including SSRIs, serotonin-norepinephrine reuptake inhibitors [SNRIs], and tetracyclic antidepressants) experience at least one adverse effect during treatment.6 Diarrhea, dizziness, dry mouth, fatigue, headache, sexual dysfunction, sweating, tremor, and weight gain are commonly reported. Nausea and vomiting are the most common reasons for discontinuation.7
EVIDENCE SUMMARY
Serious adverse effects associated with antidepressants are listed in Table 1.8–22 In the primary care setting, the number needed to harm to cause discontinuation ranged from four to 30 for TCAs and 20 to 90 for SSRIs.1 A federally funded meta-analysis concluded that discontinuation rates due to adverse effects were similar among second-generation antidepressants.6 Duloxetine (Cymbalta) and venlafaxine had slightly higher risks of discontinuation compared with SSRIs as a class (67% and 40%; 95% confidence interval, 17% to 139% and 16% to 73%, respectively).
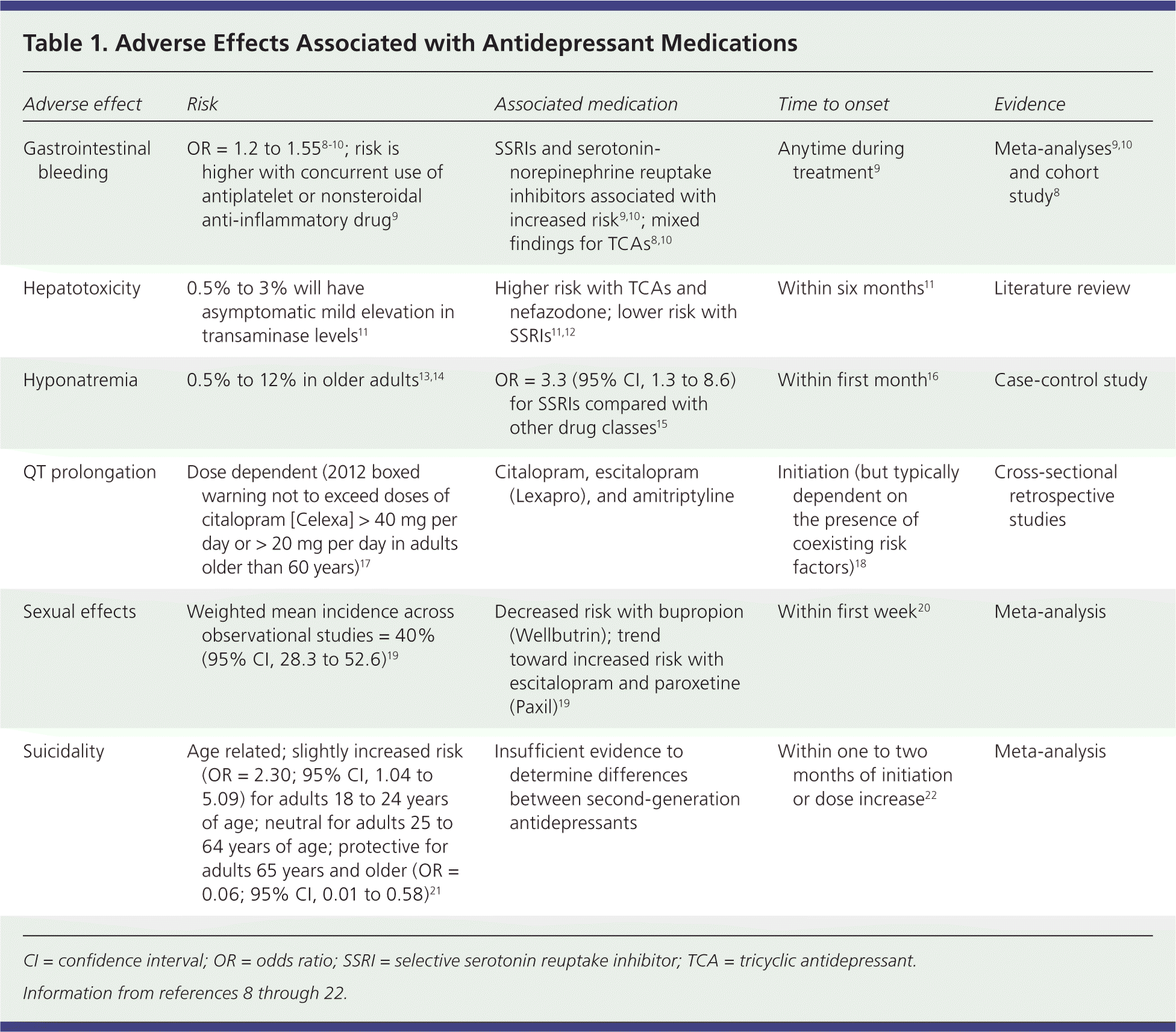
| Adverse effect | Risk | Associated medication | Time to onset | Evidence |
|---|---|---|---|---|
| Gastrointestinal bleeding | OR = 1.2 to 1.558–10; risk is higher with concurrent use of antiplatelet or nonsteroidal anti-inflammatory drug9 | SSRIs and serotonin-norepinephrine reuptake inhibitors associated with increased risk9,10; mixed findings for TCAs8,10 | Anytime during treatment9 | Meta-analyses9,10 and cohort study8 |
| Hepatotoxicity | 0.5% to 3% will have asymptomatic mild elevation in transaminase levels11 | Higher risk with TCAs and nefazodone; lower risk with SSRIs11,12 | Within six months11 | Literature review |
| Hyponatremia | 0.5% to 12% in older adults13,14 | OR = 3.3 (95% CI, 1.3 to 8.6) for SSRIs compared with other drug classes15 | Within first month16 | Case-control study |
| QT prolongation | Dose dependent (2012 boxed warning not to exceed doses of citalopram [Celexa] > 40 mg per day or > 20 mg per day in adults older than 60 years)17 | Citalopram, escitalopram (Lexapro), and amitriptyline | Initiation (but typically dependent on the presence of coexisting risk factors)18 | Cross-sectional retrospective studies |
| Sexual effects | Weighted mean incidence across observational studies = 40% (95% CI, 28.3 to 52.6)19 | Decreased risk with bupropion (Wellbutrin); trend toward increased risk with escitalopram and paroxetine (Paxil)19 | Within first week20 | Meta-analysis |
| Suicidality | Age related; slightly increased risk (OR = 2.30; 95% CI, 1.04 to 5.09) for adults 18 to 24 years of age; neutral for adults 25 to 64 years of age; protective for adults 65 years and older (OR = 0.06; 95% CI, 0.01 to 0.58)21 | Insufficient evidence to determine differences between second-generation antidepressants | Within one to two months of initiation or dose increase22 | Meta-analysis |
Overdose. One study that examined consecutive SSRI poisoning admissions to a single hospital estimated that serotonin syndrome occurs in 14% to 16% of SSRI overdoses.23 Signs of excess serotonin include tremor, diarrhea, delirium, neuromuscular rigidity, and hyperthermia. Combining SSRIs with other serotonergic medications, including some analgesics, can also cause serotonin syndrome (Table 2).24,25 Data from the National Poison Data System in 2012 showed that antidepressants trailed only analgesics and sedatives/hypnotics in toxic exposures among adults.26 SSRIs were involved in 89 fatalities, although only two were single-substance exposures, and the cause of death is unclear. TCAs were involved in 69 deaths, 17 of which were single-substance ingestions.

| Class | Drugs |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
Pregnancy. Depression during pregnancy is associated with premature birth and decreased initiation of breastfeeding.27 Antidepressant use during pregnancy has not been shown to improve these outcomes, and may increase the risk of preterm delivery compared with untreated women who have depression.28–30 SSRIs are the most commonly prescribed antidepressants for pregnant women.31 In 2005, the FDA classified paroxetine (Paxil) as pregnancy category D because of concerns about congenital cardiac malformations. More recently, however, a population-based cohort study of nearly 1 million pregnant women suggested that there is no link between first-trimester antidepressant use and cardiac malformations.32
In 2006, the FDA issued a health advisory for SSRI use after the 20th week of gestation because of concerns about increased risk of persistent pulmonary hypertension of the newborn (PPHN). This advisory was revised in 2011, and currently states that conflicting findings make it unclear whether use of SSRIs during pregnancy can cause PPHN.33 A recent meta-analysis of five trials supported the link between late pregnancy exposure to SSRIs and PPHN, with a number needed to harm of 286 to 351.34 Studies have also suggested a correlation between antidepressant use in pregnancy and lower Apgar scores, attention-deficit/hyperactivity disorder, and speech delay, although high-quality evidence is lacking.29,35,36 There are conflicting findings on the association between prenatal SSRI exposure and autism.35,37
Breastfeeding. Antidepressants transfer in low concentrations into breast milk. Paroxetine and sertraline (Zoloft) are thought to transfer in lower concentrations than other antidepressants, and produce undetectable infant plasma levels. Fluoxetine (Prozac) and venlafaxine produce the highest infant plasma concentrations. Potential adverse effects in infants exposed to SSRIs via breast milk have been documented only in case reports and are recorded more often after exposure to fluoxetine and citalopram (Celexa) than other drugs. These effects are nonspecific and include irritability and decreased feeding. There are no data on long-term neurocognitive effects. Overall, there is little evidence to support any causal link between antidepressant use in breastfeeding mothers and adverse effects in infants.38
How Do SSRIs Compare with SNRIs as First-Line Therapy?
Although SNRIs provide additional benefits to patients with comorbid pain disorders, their remission rate is only marginally superior to that of SSRIs in patients with major depressive disorder (49% vs. 42%).39–41 SNRIs are consistently associated with higher rates of adverse effects, mainly nausea and vomiting.6
EVIDENCE SUMMARY
SNRIs have been shown in meta-analyses to be superior to SSRIs for management of neuropathic pain and fibromyalgia.39,40 A systematic review found no significant difference between the drug classes for prevention of depression relapse or recurrence.6 A meta-analysis conducted in 2010 that compared SNRIs and SSRIs found a small difference in remission rates favoring SNRIs (5.7%), although dropout rates were slightly higher in the SNRI group (3.2%), suggesting only marginal differences.41 The clinical relevance of this difference is unclear.
How Should a Physician Choose a Medication for a Patient Who Has Not Previously Used Antidepressants?
Second-generation antidepressants are generally considered first-line treatment because of their better adverse effect profile, fewer serious drug interactions, and less lethality in overdose compared with TCAs and monoamine oxidase inhibitors. Because effectiveness is similar among second-generation antidepressants, agents should be chosen based on patient preferences, with adverse effect profiles, cost, and dosing frequency taken into consideration.
EVIDENCE SUMMARY
Clinical guidelines based on moderate-quality evidence recommend choosing among second-generation antidepressants based on cost, adverse effect profiles, and patient preference42 (Table 3). For patients with accompanying anxiety, there are no conclusive data supporting the use of one agent over another. For patients with accompanying insomnia, SSRIs appear to have similar effectiveness, but some studies have shown trazodone and nefazodone to be superior. Mirtazapine (Remeron) has a faster onset of action than some SSRIs, but it is associated with long-term weight gain.12 Sertraline has a higher incidence of diarrhea than most other second-generation antidepressants.6 Other adverse effects are listed in Table 1.8–22
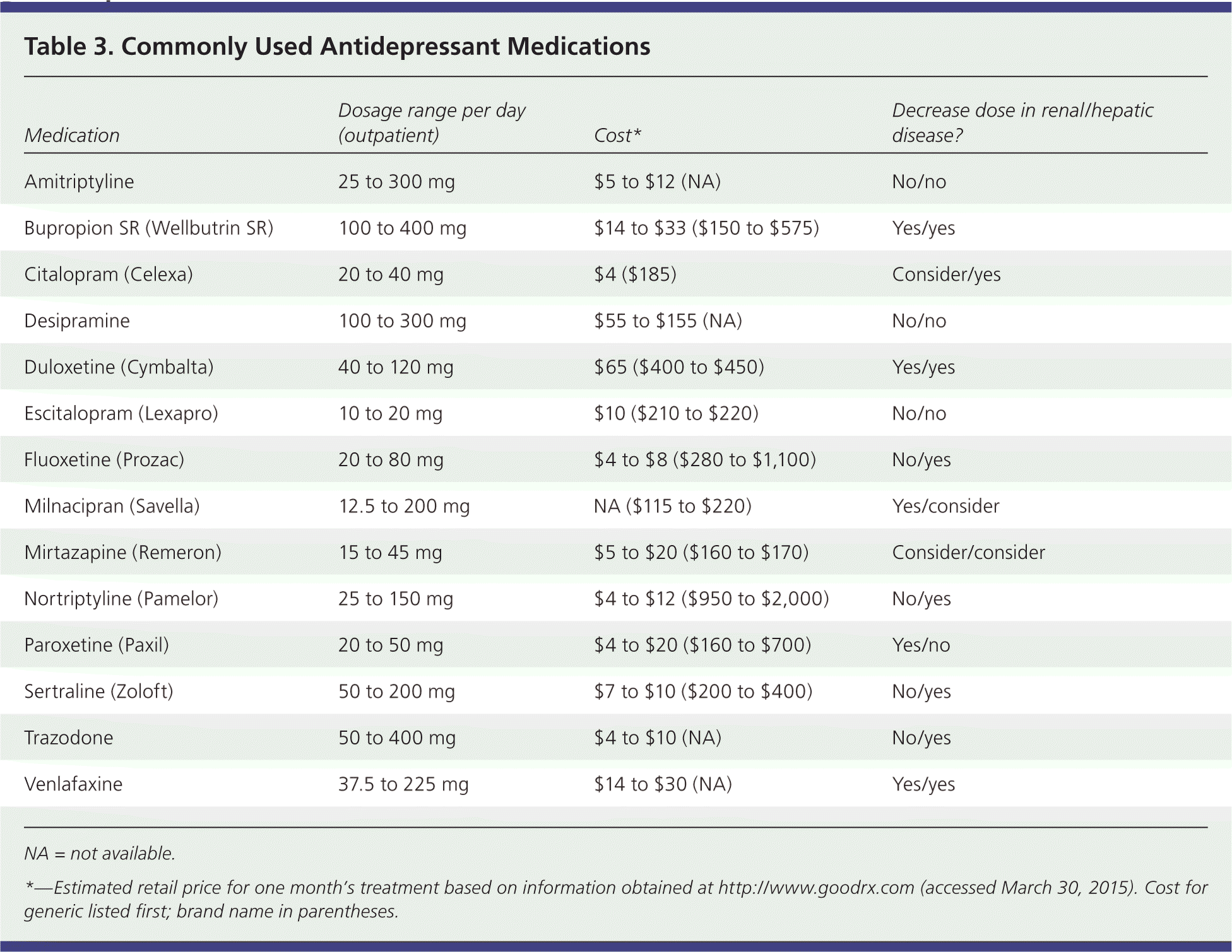
| Medication | Dosage range per day (outpatient) | Cost* | Decrease dose in renal/hepatic disease? |
|---|---|---|---|
| Amitriptyline | 25 to 300 mg | $5 to $12 (NA) | No/no |
| Bupropion SR (Wellbutrin SR) | 100 to 400 mg | $14 to $33 ($150 to $575) | Yes/yes |
| Citalopram (Celexa) | 20 to 40 mg | $4 ($185) | Consider/yes |
| Desipramine | 100 to 300 mg | $55 to $155 (NA) | No/no |
| Duloxetine (Cymbalta) | 40 to 120 mg | $65 ($400 to $450) | Yes/yes |
| Escitalopram (Lexapro) | 10 to 20 mg | $10 ($210 to $220) | No/no |
| Fluoxetine (Prozac) | 20 to 80 mg | $4 to $8 ($280 to $1,100) | No/yes |
| Milnacipran (Savella) | 12.5 to 200 mg | NA ($115 to $220) | Yes/consider |
| Mirtazapine (Remeron) | 15 to 45 mg | $5 to $20 ($160 to $170) | Consider/consider |
| Nortriptyline (Pamelor) | 25 to 150 mg | $4 to $12 ($950 to $2,000) | No/yes |
| Paroxetine (Paxil) | 20 to 50 mg | $4 to $20 ($160 to $700) | Yes/no |
| Sertraline (Zoloft) | 50 to 200 mg | $7 to $10 ($200 to $400) | No/yes |
| Trazodone | 50 to 400 mg | $4 to $10 (NA) | No/yes |
| Venlafaxine | 37.5 to 225 mg | $14 to $30 (NA) | Yes/yes |
Which Patients Are Most Likely to Respond to Antidepressant Medication?
Antidepressant medication has greater benefit in patients with severe depression compared with placebo.
EVIDENCE SUMMARY
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial studied 2,876 adults 18 to 75 years of age who were treated for nonpsychotic depression in the psychiatric or primary care setting.43 It found that the patients most likely to achieve remission while taking citalopram were white women who were employed and had higher education or income levels and shorter episode duration. Higher baseline depression severity was associated with a lower likelihood of achieving remission with medication. Because the STAR*D trial did not include a placebo arm, it is difficult to determine how many patients who achieved remission with citalopram would have experienced the same outcome with alternative therapy or no therapy at all.43
Two meta-analyses of published and unpublished studies submitted to the FDA concluded that antidepressants were superior to placebo in patients with more severe depression, and that there was little difference between drug therapy and placebo in patients with less severe depression.44,45 Patients with severe depression did not respond as well to placebo. A patient-level meta-analysis of six trials found that patients with severe depression had more robust responses to antidepressants compared with placebo than did patients with mild to moderate depression.46
Are There Special Considerations for Pharmacologic Treatment in Older Adults?
Consensus guidelines recommend a “start low, go slow” approach to antidepressant therapy in older adults. Preferred agents include citalopram, escitalopram (Lexapro), sertraline, mirtazapine, venlafaxine, and bupropion (Wellbutrin).
EVIDENCE SUMMARY
Because older adults are at significantly greater risk of adverse drug reactions compared with younger populations,47 lower starting doses are often recommended (i.e., approximately 50% of the adult starting dose). A Cochrane meta-analysis comparing SSRIs with TCAs in older patients reported no difference in effectiveness between the two drug classes, but noted that patients receiving TCAs were more likely to withdraw from the study and discontinue drug therapy because of adverse reactions.48 Tertiary-amine TCAs (e.g., amitriptyline, imipramine [Tofranil]) are associated with significant adverse anticholinergic effects and are considered a potentially inappropriate medication in the American Geriatric Society's Beers Criteria.49 Secondary-amine TCAs (e.g., nortriptyline [Pamelor], desipramine) are thought to be safer because of their lower affinity for muscarinic receptor antagonism.50
Guidelines recommend several SSRIs (e.g., citalopram, escitalopram, sertraline) as good first-line options.50 Bupropion, mirtazapine, and venlafaxine are also considered appropriate because of their favorable adverse effect profiles. Paroxetine is associated with more anticholinergic effects, and fluoxetine has a greater risk of agitation and overstimulation; neither should be used in older adults.50
What Is the Optimal Duration of Antidepressant Treatment?
EVIDENCE SUMMARY
After a first episode of depression, the probability of a recurrent episode is approximately 50%; this probability increases to 70% after two episodes and to 90% after a third episode.52 Antidepressant medication does not prevent relapse if it is discontinued at the end of the acute phase.52 Systematic reviews show that continued treatment with antidepressants after remission protects against recurrence and relapse.53,54 Most studies of antidepressant medications are conducted for one year or less,55 but more than 60% of Americans on antidepressant therapy have been taking it longer than two years, and 14% have done so for 10 years or longer.56 A randomized controlled trial enrolled patients with recurrent depression (defined as three or more episodes) who were treated to remission with venlafaxine or fluoxetine for six months.57 The venlafaxine-treated group was then randomized to maintenance venlafaxine or placebo for 12 months, after which those with sustained remission in the venlafaxine arm were rerandomized for another 12 months. Those treated with fluoxetine were continued on fluoxetine for both maintenance phases. After both follow-up periods, depression recurrence rates were significantly lower in patients treated with fluoxetine or venlafaxine compared with those who received placebo.
Data Sources: A PubMed search was completed in Clinical Queries using the key terms depression, antidepressant, safety, efficacy, overdose, serotonin syndrome, pregnancy, breastfeeding, and duration. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Highly cited studies found within review articles and major databases were also reviewed and included as appropriate. Also searched were the Agency for Healthcare Research and Quality evidence reports, the Cochrane database, Essential Evidence Plus, and the National Guideline Clearinghouse database. Search dates: June 2014 through April 2015.
