
Am Fam Physician. 2009;79(11):985-992
A more recent article on first trimester bleeding is available.
Patient information: See related handout on first trimester bleeding, written by the authors of this article.
Author disclosure: Nothing to disclose.
Vaginal bleeding in the first trimester occurs in about one fourth of pregnancies. About one half of those who bleed will miscarry. Guarded reassurance and watchful waiting are appropriate if fetal heart sounds are detected, if the patient is medically stable, and if there is no adnexal mass or clinical sign of intraperitoneal bleeding. Discriminatory criteria using transvaginal ultrasonography and beta subunit of human chorionic gonadotropin testing aid in distinguishing among the many conditions of first trimester bleeding. Possible causes of bleeding include subchorionic hemorrhage, embryonic demise, anembryonic pregnancy, incomplete abortion, ectopic pregnancy, and gestational trophoblastic disease. When beta subunit of human chorionic gonadotropin reaches levels of 1,500 to 2,000 mIU per mL (1,500 to 2,000 IU per L), a normal pregnancy should exhibit a gestational sac by transvaginal ultrasonography. When the gestational sac is greater than 10 mm in diameter, a yolk sac must be present. A live embryo must exhibit cardiac activity when the crown-rump length is greater than 5 mm. In a normal pregnancy, beta subunit of human chorionic gonadotropin levels increase by 80 percent every 48 hours. The absence of any normal discriminatory findings is consistent with early pregnancy failure, but does not distinguish between ectopic pregnancy and failed intrauterine pregnancy. The presence of an adnexal mass or free pelvic fluid represents ectopic pregnancy until proven otherwise. Medical management with misoprostol is highly effective for early intrauterine pregnancy failure with the exception of gestational trophoblastic disease, which must be surgically evacuated. Expectant treatment is effective for many patients with incomplete abortion. Medical management with methotrexate is highly effective for properly selected patients with ectopic pregnancy. Follow-up after early pregnancy loss should include attention to future pregnancy planning, contraception, and psychological aspects of care.
About one fourth of all pregnant women experience spotting or bleeding in the first several weeks of pregnancy, and one half of those who bleed will miscarry.1 Family physicians who are familiar with the normal progression of early pregnancy anatomy, sonographic findings, and beta subunit of human chorionic gonadotropin (β-hCG) values can make a definitive diagnosis and proceed with appropriate treatment.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Evidence does not support the routine use of antibiotics in all women with incomplete abortion. | A | 5 |
| A normal pregnancy should exhibit a gestational sac when beta subunit of human chorionic gonadotropin levels reach 1,500 to 2,000 mIU per mL (1,500 to 2,000 IU per L), a yolk sac when the gestational sac is greater than 10 mm in diameter, and cardiac activity when the embryonic crown-rump length is greater than 5 mm. | C | 1 |
| Because expectant and surgical management of miscarriage are equally effective, the patient's preference should play a dominant role in choosing a treatment. | C | 16 |
| When the patient has an incomplete abortion, nonsurgical treatments have a high likelihood of success; when the patient has an embryonic demise or anembryonic pregnancy, misoprostol (Cytotec) or surgical treatment is more effective than expectant treatment. | A | 13–15 |
| Vaginal misoprostol is safer and more effective than oral misoprostol, with fewer gastrointestinal side effects. | A | 13, 15 |
| After a first trimester pregnancy loss, patients who are Rh negative should receive 50 mcg of anti D immune globulin. | C | 27 |
| Acknowledgment of grief, sympathy, and reassurance are useful techniques in counseling patients after miscarriage. | C | 31 |
Managing First Trimester Bleeding
By definition, bleeding before 20 weeks of gestation constitutes threatened abortion (Table 12,3), but the majority of such pregnancies progress normally. The pace of evaluation depends on the patient's history, signs, and symptoms. If known, the time since the patient's last normal menses may be used to estimate the gestational age. In stable patients, the physical examination includes documentation of the size and position of the uterus, auscultation of fetal heart sounds by Doppler (if it has been at least 10 to 11 weeks since last normal menses), and bimanual examination for masses and tenderness. Guarded reassurance and watchful waiting are appropriate if fetal heart sounds are detected with Doppler, if the patient is stable, and if there is no adnexal mass, significant tenderness, or clinical sign of intraperitoneal bleeding.
| Term | Definition | |
|---|---|---|
| Anembryonic pregnancy | Presence of a gestational sac larger than 18 mm without evidence of embryonic tissues (yolk sac or embryo); this term is preferable to the older and less accurate term “blighted ovum” | |
| Ectopic pregnancy | Pregnancy outside the uterine cavity (most commonly in the fallopian tube) but may occur in the broad ligament, ovary, cervix, or elsewhere in the abdomen | |
| Embryonic demise | An embryo larger than 5 mm without cardiac activity; this replaces the term “missed abortion” | |
| Gestational trophoblastic disease or hydatidiform mole | Complete mole: placental proliferation in the absence of a fetus; most have a 46, XX chromosomal composition; all derived from paternal source | |
| Partial mole: molar placenta occurring with a fetus; most are genetically triploid (69, XXY) | ||
| Heterotopic pregnancy | Simultaneous intrauterine and ectopic pregnancy; risk factors include ovulation induction, in vitro fertilization, and gamete intrafallopian transfer | |
| Recurrent pregnancy loss | More than two consecutive pregnancy losses; “habitual aborter” has also been used but is no longer appropriate | |
| Spontaneous abortion | Spontaneous loss of a pregnancy before 20 weeks' gestation | |
| Complete abortion | Complete passage of all products of conception | |
| Incomplete abortion | Occurs when some, but not all, of the products of conception have passed | |
| Inevitable abortion | Bleeding in the presence of a dilated cervix; indicates that passage of the conceptus is unavoidable | |
| Septic abortion | Incomplete abortion associated with ascending infection of the endometrium, parametrium, adnexa, or peritoneum | |
| Subchorionic hemorrhage | Sonographic finding of blood between the chorion and uterine wall, usually in the setting of vaginal bleeding | |
| Threatened abortion | Bleeding before 20 weeks' gestation in the presence of an embryo with cardiac activity and closed cervix | |
| Vanishing twin | A multifetal pregnancy is identified and one or more fetuses later disappear (occurs more often now that early ultrasonography is common); if early in pregnancy, the embryo is often reabsorbed; if later in pregnancy, it leads to a compressed or mummified fetus or amorphous material | |
Adnexal tenderness and the presence of a mass may indicate ectopic pregnancy. Hypotension with other symptoms of hemoperitoneum (e.g., shoulder pain, absent bowel sounds, distended doughy abdomen) may point to ruptured ectopic pregnancy and prompt immediate hospitalization for evaluation.
Examination with a vaginal speculum may reveal nonobstetric causes of bleeding, such as cervicitis, vaginitis, cystitis, trauma, cervical cancer, or polyps; or nonvaginal causes of bleeding, such as hemorrhoids. Significant cervical dilation or visible products of conception are indicative of an inevitable abortion. Tissue may be removed by gentle traction with ring forceps, and may be examined for the presence of chorionic villi (Figure 1) or sent for pathologic examination. Chorionic villi are indicative of spontaneous abortion. Table 2 shows risk factors of spontaneous abortion.2–4
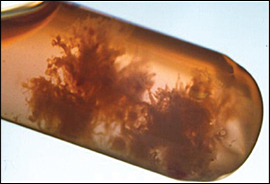
| Spontaneous abortion |
| Endocrine (e.g., progesterone deficiency, thyroid disease, uncontrolled diabetes) |
| Genetic aneuploidy (accounts for about one half of spontaneous abortions) |
| Immunologic (e.g., antiphospholipid syndrome, lupus) |
| Infection (e.g., chlamydia, gonorrhea, herpes, listeria, mycoplasma, syphilis, toxoplasmosis, ureaplasma) |
| Occupational chemical exposure |
| Radiation exposure |
| Uterine (e.g., congenital anomalies) |
| Ectopic pregnancy |
| Current intrauterine device |
| History of ectopic pregnancy |
| History of in utero exposure to diethylstilbestrol |
| History of genital infection, including pelvic inflammatory disease, chlamydia, or gonorrhea |
| History of tubal surgery, including tubal ligation or reanastomosis of the tubes after tubal ligation |
| In vitro fertilization |
| Infertility |
| Smoking |
Cervical testing for gonorrhea and chlamydia may be performed. Fever and significant adnexal or peritoneal symptoms are found in septic abortion. Treatment of septic abortion is urgent, including prompt antibiotic administration and uterine evacuation. Infection, retained products of conception, and uterine perforation are more common if the history includes attempted abortion by someone who is untrained. Evidence does not support the routine use of antibiotics in all women with incomplete abortion.5
If history and physical examination do not yield a diagnosis, ultrasonography with or without β-hCG testing is required. Application and interpretation of this testing will help determine the differential diagnosis of early pregnancy failure.
Normal First Trimester Pregnancy Markers
HUMAN CHORIONIC GONADOTROPIN
The first measurable finding in pregnancy is an elevated β-hCG level, which is produced by the placenta after implantation of the blastocyst. This occurs at approximately 23 menstrual days' gestation, or as early as eight days after conception. Readily available home urine pregnancy tests detect β-hCG levels as low as 25 mIU per mL (25 IU per L) International Reference Preparation. Therefore, it is possible to diagnose pregnancy before a missed period.6
Quantitative serum β-hCG levels rise in a predictable fashion during the first four to eight weeks of normal pregnancy, increasing by 80 percent every 48 hours. This rate of increase in β-hCG levels is reassuring, but not indicative of normal pregnancy. Inadequately rising β-hCG levels do not distinguish between ectopic and failing intrauterine pregnancy. Unexpectedly high β-hCG levels require consideration of gestational trophoblastic disease.
ULTRASONOGRAPHY
Early detection of pregnancy depends on transvaginal ultrasonography using transducer frequency of 5 MHz or greater.1 A 5-mm sonolucent gestational sac should be visible in the endometrium at the fundus by five menstrual weeks.1 The normal sac of an intrauterine pregnancy consists of a central blastocyst surrounded by a double ring of echogenic chorionic villi and decidua. This distinguishes it from a pseudogestational sac associated with ectopic pregnancy.
The yolk sac is visible using transvaginal scanning by six menstrual weeks. This confirms an intrauterine pregnancy (Figure 2). By the end of the sixth week, a 2- to 5-mm embryo or fetal pole becomes visible (Figure 3). Measurement of the embryonic crown-rump length is the most accurate way to date pregnancy (Figure 4). Cardiac activity should be present when the embryo exceeds 5 mm in length.1 Transabdominal scanning is less sensitive, and will show these landmarks about one week after they are visible transvaginally.
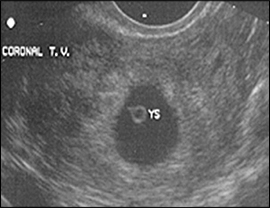
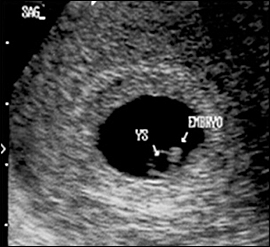
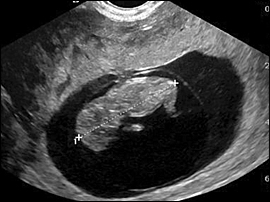
DISCRIMINATORY CRITERIA
The predictable, linked progression of laboratory and sonographic findings constitutes discriminatory criteria, as shown in Table 3.1 A normal pregnancy should exhibit a gestational sac when β-hCG levels reach 1,500 to 2,000 mIU per mL (1,500 to 2,000 IU per L), a yolk sac when the gestational sac is greater than 10 mm in diameter, and cardiac activity when the crown-rump length is greater than 5 mm.1 The absence of an expected discriminatory finding is consistent with pregnancy failure; however, because of the emotional impact of pregnancy loss, imaging may be repeated one week later to confirm the diagnosis. After an embryo with a heartbeat is confirmed using these criteria, continued follow-up by ultrasonography provides more specific information about the state of the pregnancy than serial measurements of β-hCG.
Making a Diagnosis
| Menstrual age | Embryologic event | Laboratory and transvaginal sonographic discriminatory findings |
|---|---|---|
| Three to four weeks | Implantation site | Decidual thickening |
| Four weeks | Trophoblast | Peritrophoblastic flow on color flow Doppler |
| Four to five weeks | Gestational sac | Present when beta subunit of human chorionic gonadotropin level is greater than 1,500 to 2,000 mIU per mL (1,500 to 2,000 IU per L; varies with sonographer experience and quality of ultrasonography) |
| Five to six weeks | Yolk sac | Present when diameter of gestational sac is greater than 10 mm |
| Five to six weeks | Embryo | Present when diameter of gestational sac is greater than 18 mm |
| Five to six weeks | Cardiac activity | Present when embryonic crown-rump length is greater than 5 mm |
ECTOPIC PREGNANCY
Ectopic pregnancy is responsible for 6 percent of all U.S. maternal deaths.4 Intrauterine pregnancy with yolk sac or embryo rules out ectopic pregnancy, with the exception of a one in 4,000 chance of heterotopic pregnancy. Transvaginal ultrasonography demonstrates an intra-uterine gestational sac with nearly 100 percent sensitivity at β-hCG levels of 1,500 to 2,000 mIU per mL.1 If the β-hCG level is above this discriminatory cutoff and a gestational sac is not visible, then there is a high likelihood of ectopic pregnancy.
An embryo with cardiac activity outside the uterus proves ectopic pregnancy. Conditions that impede the tubal transport of a fertilized ovum are associated with ectopic pregnancy7 (Table 22–4), although many ectopic pregnancies occur in women without risk factors. Early diagnosis is the key to preventing morbidity and mortality, and preserving fertility.
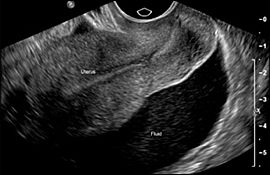
An adnexal mass or free pelvic fluid (Figure 5) signifies a high probability of ectopic pregnancy, even if the gestational sac or embryo is not visible. The presence of a corpus luteum cyst of pregnancy may confuse the picture.
SPONTANEOUS ABORTION AND EMBRYONIC DEMISE
Products of conception in the cervix or an intrauterine embryo without cardiac activity proves incomplete abortion, inevitable abortion, or embryonic demise. Their presence rules out ectopic pregnancy, although there is a one in 4,000 chance of heterotopic pregnancy. Anembryonic pregnancy is often suspected when the patient reports regression of pregnancy symptoms or when Doppler fails to detect fetal heart sounds by 10 to 11 weeks after the last normal menses.
GESTATIONAL TROPHOBLASTIC DISEASE
SUBCHORIONIC HEMORRHAGE
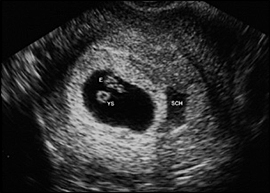
Subchorionic hemorrhage (Figure 6) is a common finding with first trimester bleeding and may also be an incidental finding in uncomplicated pregnancies. It is important to note whether embryonic cardiac activity is present. Subchorionic hemorrhage may be mistaken for a twin gestational sac.
THE DIFFICULT DIAGNOSIS
It is common for β-hCG levels to be less than 1,500 mIU per mL with sonographic findings that are nondiagnostic. It is reasonable to perform repeat ultrasonography after one week in a stable patient. This interval allows significant growth of the gestational sac or embryo, both of which should grow at the rate of 1 mm per day. In this situation, serial quantitative β-hCG levels in combination with follow-up imaging are also useful. Based on a prospective study, the minimum β-hCG increase necessary for a living pregnancy over a 48-hour interval is 53 percent.10 When β-hCG rises more slowly than this, plateaus, or falls, the differential diagnosis is limited to failing intrauterine pregnancy or ectopic pregnancy.
When β-hCG levels are not rising normally and ultrasonography cannot confirm pregnancy location, a dilatation and curettage or manual vacuum aspiration may be helpful. Manual vacuum aspiration requires a specially-designed 60-mL syringe with attached cannula to apply suction to the uterine cavity. If evacuation of the uterus yields chorionic villi, then a failed intrauterine pregnancy is diagnosed and treatment for an ectopic pregnancy may be avoided. When ectopic pregnancy is suspected but cannot be confirmed with noninvasive testing, consultation for diagnostic laparoscopy or treatment with methotrexate is appropriate.
Management
THREATENED ABORTION
The presence of an intrauterine embryo with cardiac activity on ultrasonography should be reassuring because it essentially rules out ectopic pregnancy. It is also associated with a pregnancy loss rate of only 2 percent in women 35 years and younger and 16 percent in women older than 35 years.11 If subchorionic hemorrhage (Figure 6) is present in an intrauterine pregnancy with fetal heart sounds, the likelihood of spontaneous abortion is 9 percent and may be even higher if the patient is older than 35 years or if the hematoma is large.12 In this situation, the physician should caution patients to expect continued bleeding and possible impending miscarriage.
SPONTANEOUS ABORTION, EMBRYONIC DEMISE, AND ANEMBRYONIC PREGNANCY
Most first trimester miscarriages occur completely and spontaneously without intervention. Although dilatation and curettage has historically been the treatment of choice, several recent trials confirm that expectant management or medical management with misoprostol (Cytotec) can be as effective and safer while offering the patient more control over her care.13–16
Clinical trials comparing expectant, medical, and surgical management reach several conclusions. In incomplete abortion, high success rates have been demonstrated for expectant management (86 percent) and medical management (100 percent).13 However, expectant management is more likely to fail in embryonic demise or anembryonic pregnancy; in these patients, the success of expectant management by day 7 drops to 29 percent, compared with 87 percent for medical management.13 Women treated expectantly have more outpatient visits than those treated medically.13 Women treated medically have more bleeding but less pain than those treated surgically.14 Surgery is associated with more trauma and infectious complications than medical treatment.14,16 Misoprostol has fewer gastrointestinal side effects when given vaginally than when given orally.13,15
Based on these findings, increased use of medical management has benefits for patients, although misoprostol is not approved by the U.S. Food and Drug Administration for use in treating miscarriage.17 The doses used in published studies are 800 mcg vaginally or 600 mcg orally.13–15 If a single vaginal dose of 800 mcg does not result in complete expulsion by day 3, the dose should be repeated. If complete expulsion has not occurred by day 8, manual vacuum aspiration should be offered.18 Additional published protocols are available.19
Confirming a negative urine β-hCG four to six weeks after early pregnancy loss excludes the presence of persistent gestational trophoblastic disease.20 Although there is solid evidence to support the use of prophylactic antibiotics for induced abortion, there is no evidence supporting this practice in early pregnancy failure.21
ECTOPIC PREGNANCY
Early diagnosis of ectopic pregnancy brings management into the outpatient setting. Current treatment options favor medical and laparoscopic management,22 with expectant management reserved for patients with a declining quantitative β-hCG of less than 1,000 mIU per mL (1,000 IU per L) and open surgical management reserved for patients with tubal rupture and hemoperitoneum. Medical management with methotrexate is appropriate for properly selected patients. Randomized trials have shown medical management to be safer, more effective, and less expensive than conservative surgical treatment, and to result in equal or better subsequent fertility.23-26 Ectopic pregnancy management options, including methotrexate dosages and follow-up criteria, are shown in Table 4.23–26
| Expectant management |
| No evidence of tubal rupture |
| Minimal pain or bleeding |
| Patient reliable for follow-up |
| Starting β-hCG level less than 1,000 mIU per mL (1,000 IU per L) and falling |
| Ectopic or adnexal mass less than 3 cm or not detected |
| No embryonic heartbeat |
| Medical management with methotrexate |
| Stable vital signs and few symptoms |
| No medical contraindication for methotrexate therapy (e.g., normal liver enzymes, complete blood count and platelet count) |
| Unruptured ectopic pregnancy |
| Absence of embryonic cardiac activity |
| Ectopic mass of 3.5 cm or less |
| Starting β-hCG levels less than 5,000 mIU per mL (5,000 IU per L) |
| Dosage: single intramuscular dose of 1 mg per kg, or 50 mg per m2 |
| Follow-up: β-hCG on the fourth and seventh posttreatment days, then weekly until undetectable, which usually takes several weeks |
| Expected β-hCG changes: initial slight increase, then 15 percent decrease between days 4 and 7; if not, repeat dosage or move to surgery |
| Special consideration: prompt availability of surgery if patient does not respond to treatment |
| Surgical management |
| Unstable vital signs or signs of hemoperitoneum |
| Uncertain diagnosis |
| Advanced ectopic pregnancy (e.g., high β-hCG levels, large mass, cardiac activity) |
| Patient unreliable for follow-up |
| Contraindications to observation or methotrexate |
Care After Pregnancy Loss
Several follow-up issues must be addressed after any type of pregnancy loss. Women who are Rh negative and miscarry during the first trimester should receive 50 mcg of anti D immune globulin.27 Contraception should be discussed and started immediately. All methods are equally safe immediately following spontaneous abortion or ectopic pregnancy. There is no good evidence suggesting an ideal interpregnancy interval,28 but folic acid supplementation before attempts at future conception substantially reduces the risk of neural tube defects.29 The psychological impact of pregnancy loss can be devastating to the patient and her partner.30 Approaches to managing patient grief are shown in Table 5.31
| Acknowledge and attempt to dispel guilt | |
| Acknowledge and legitimize grief | |
| Provide comfort, sympathy, and ongoing support | |
| Reassure the patient about the future | |
| Counsel the patient on how to tell family and friends about the miscarriage: | |
| Speak simply and honestly | |
| Avoid medical details | |
| Recognize that others may react emotionally | |
| Explain how others can help, if known | |
| Warn patients of the anniversary phenomenon | |
| Include the patient's partner in your psychological care | |
| Assess level of grief and adjust counseling accordingly | |