Am Fam Physician. 2017;95(2):78-87
Related editorial: The Cholesterol Dilemma: Treating the Risk or Treating to LDL-C Goal?
Related U.S. Preventive Services Task Force recommendation statement: Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: Recommendation Statement
Author disclosure: No relevant financial affiliations.
Guidelines from the American College of Cardiology/American Heart Association (ACC/AHA) and the U.K. National Institute for Health and Care Excellence (NICE) indicate that lipid-lowering drugs have benefit for primary and secondary prevention of atherosclerotic cardiovascular disease (ASCVD) events. The evidence is strongest for statins. ACC/AHA, NICE, and U.S. Preventive Services Task Force (USPSTF) guidelines recommend statin therapy based on patients' risk of an ASCVD event, rather than treating to specific lipid levels. For patients with no previous ASCVD event, risk calculators should be used to determine the 10-year risk of ASCVD. The ACC/AHA guideline recommends starting moderate- to high-intensity statins if the risk is 7.5% or greater, whereas the NICE and USPSTF guidelines recommend statins if the risk is 10% or greater. Patients with known ASCVD should receive high-intensity statins unless they fall into special categories (e.g., older age) or do not tolerate high-intensity statins, in which case moderate-intensity statins are appropriate. Liver transaminase levels should be checked before starting statins; guidelines vary on if and when to recheck them in the absence of symptoms. Lipid levels should be rechecked one to three months after starting statins, although guidelines differ on subsequent checks. Other lipid-lowering drugs (e.g., bile acid sequestrants, ezetimibe) can be considered if patients do not tolerate statins. Niacin should not be used. Some evidence supports adding ezetimibe to statin therapy in patients with acute coronary syndrome or chronic kidney disease. The role of proprotein convertase subtilisin/kexin type 9 inhibitors is unclear, but initial studies suggest a decrease in the rate of acute ASCVD events in patients with hypercholesterolemia.
The American College of Cardiology (ACC) and the American Heart Association (AHA) jointly issued a new guideline in 2013 on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults.1 This guideline presented significant changes to previous recommendations on the management of hyperlipidemia for primary and secondary prevention of atherosclerotic cardiovascular disease (ASCVD), which includes acute coronary syndrome, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, and peripheral arterial disease presumed to be of atherosclerotic origin.
WHAT IS NEW ON THIS TOPIC: HYPERLIPIDEMIA
A Cochrane review found that for every 1,000 persons treated with a statin for five years, 18 avoid myocardial infarction, angina, or stroke (number needed to treat = 56). This review focused on studies that included fewer than 10% of participants with known cardiovascular disease.
A meta-analysis of 13 trials involving more than 90,000 patients found that statin use increases the overall absolute risk of developing diabetes mellitus by 0.39% (number needed to harm = 255) over four years.
In early 2016, the U.S. Food and Drug Administration withdrew approvals for extended-release niacin and delayed-release fenofibric acid (fibrate) in combination with statins based on lack of cardiovascular benefit.
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Patients with a high risk of ASCVD (a 10-year risk of at least 7.5% or 10%, depending on the guideline) should receive statin therapy for primary prevention. | B | 1, 3, 11, 17, 18 | Inconsistent results from meta-analyses regarding benefit in low-risk patients |
| Statin therapy should be prescribed for secondary prevention in patients with known ASCVD, unless contraindicated. | A | 1, 2, 24–28 | Recommendation from consensus guidelines and meta-analyses |
| Niacin, fibrates, and omega-3 fatty acids should not be routinely prescribed for primary or secondary prevention of ASCVD. Although these agents lower cholesterol levels, they do not affect patient-oriented outcomes. | A | 1, 20, 32–40 | Consistent results in meta-analyses and systematic review |
| A moderate-intensity statin plus ezetimibe should be considered as an alternative in patients with acute coronary syndrome who do not tolerate high-intensity statin therapy. | B | 31 | One randomized controlled trial with high discontinuation rates |
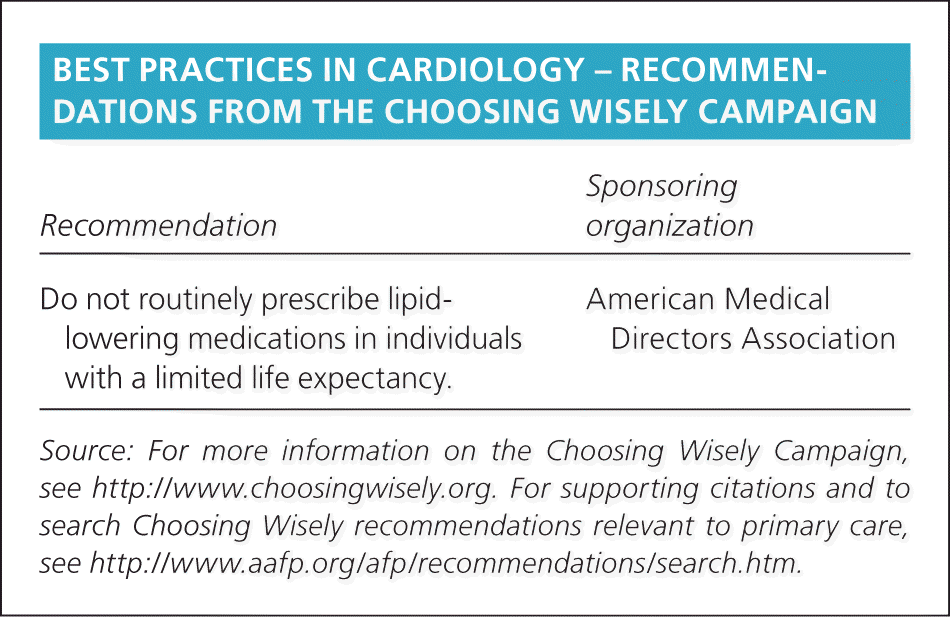
| Recommendation | Sponsoring organization |
|---|---|
| Do not routinely prescribe lipid-lowering medications in individuals with a limited life expectancy. | American Medical Directors Association |
Recommendations and guidelines from the ACC/AHA, U.K. National Institute for Health and Care Excellence (NICE), and U.S. Preventive Services Task Force (USPSTF) have eliminated goals for low-density lipoprotein cholesterol (LDL-C) and non–high-density lipoprotein cholesterol levels because studies to date have focused on treatment intensity rather than cholesterol levels (Table 1).1–4 As a result of this shift, the ACC/AHA and NICE guidelines recommend a different intensity of statin therapy based on ASCVD risk (Table 2).1 The USPSTF recommends using low- to moderate-intensity statins only in patients with a combination of at least one ASCVD risk factor and a 10-year ASCVD risk of at least 10%.3 The NICE guideline also uses a 10-year ASCVD risk of 10% or greater as the trigger for statin therapy, whereas the ACC/AHA guideline recommends a threshold of 7.5% or greater (Table 1).1–4 The ACC/AHA also recommends that patients with a 10-year risk between 5.0% and 7.5% be considered for statin therapy. Recommendations for statin initiation based on these guidelines are shown in Figure 1.1–3
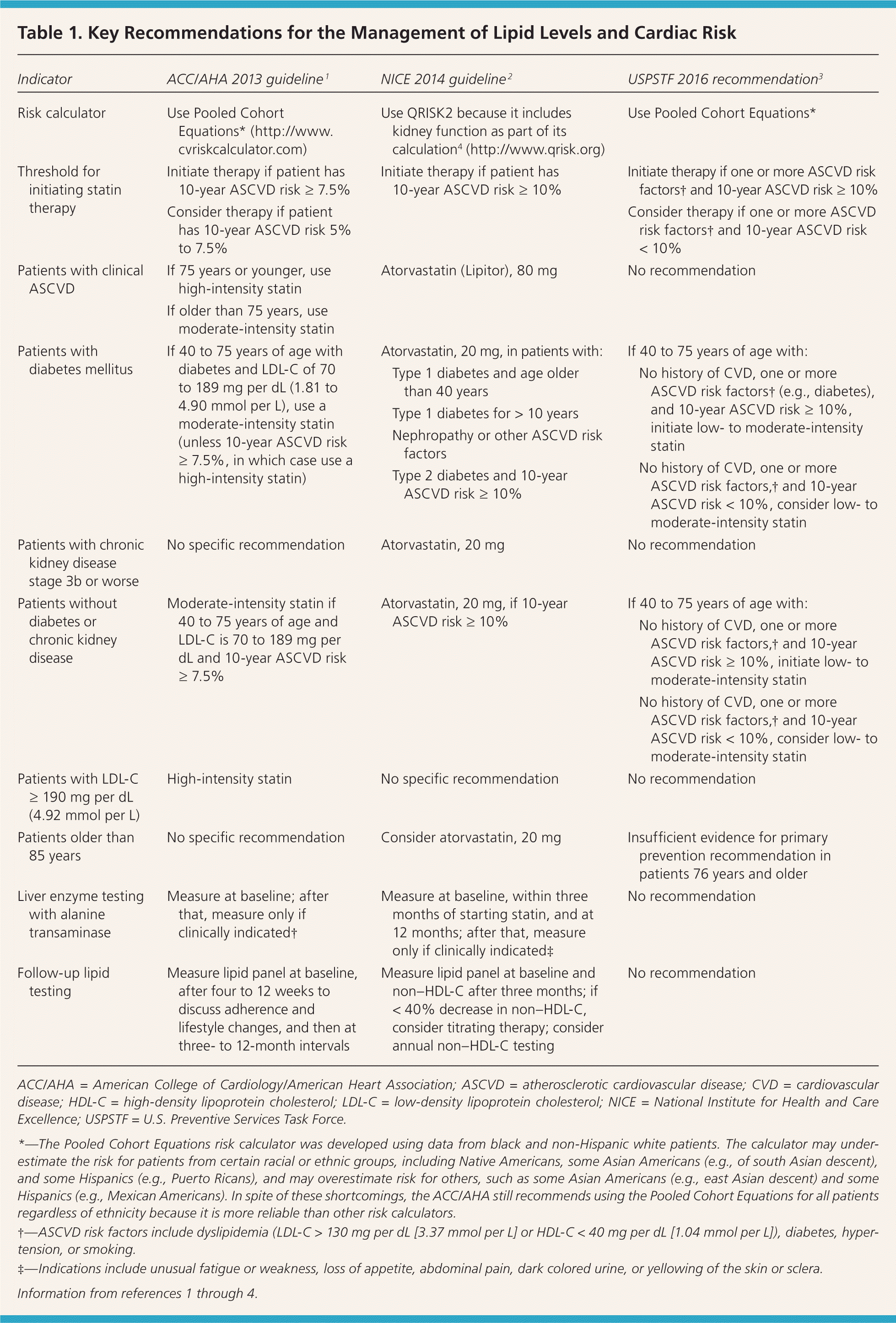
| Indicator | ACC/AHA 2013 guideline 1 | NICE 2014 guideline 2 | USPSTF 2016 recommendation3 |
|---|---|---|---|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
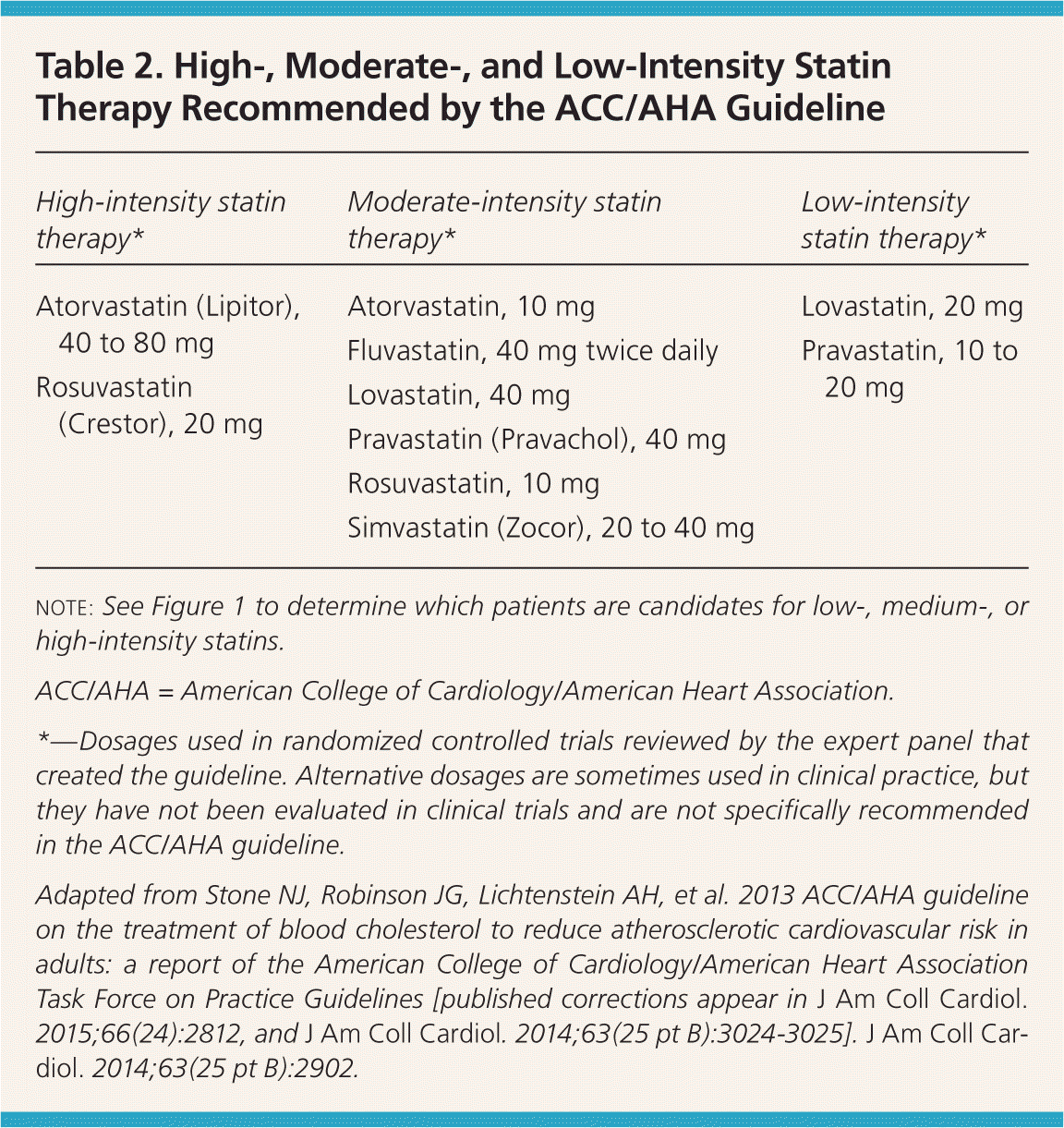
| High-intensity statin therapy* | Moderate-intensity statin therapy* | Low-intensity statin therapy* |
|---|---|---|
| Atorvastatin (Lipitor), 40 to 80 mg | Atorvastatin, 10 mg | Lovastatin, 20 mg |
| Rosuvastatin (Crestor), 20 mg | Fluvastatin, 40 mg twice daily | Pravastatin, 10 to 20 mg |
| Lovastatin, 40 mg | ||
| Pravastatin (Pravachol), 40 mg | ||
| Rosuvastatin, 10 mg | ||
| Simvastatin (Zocor), 20 to 40 mg |
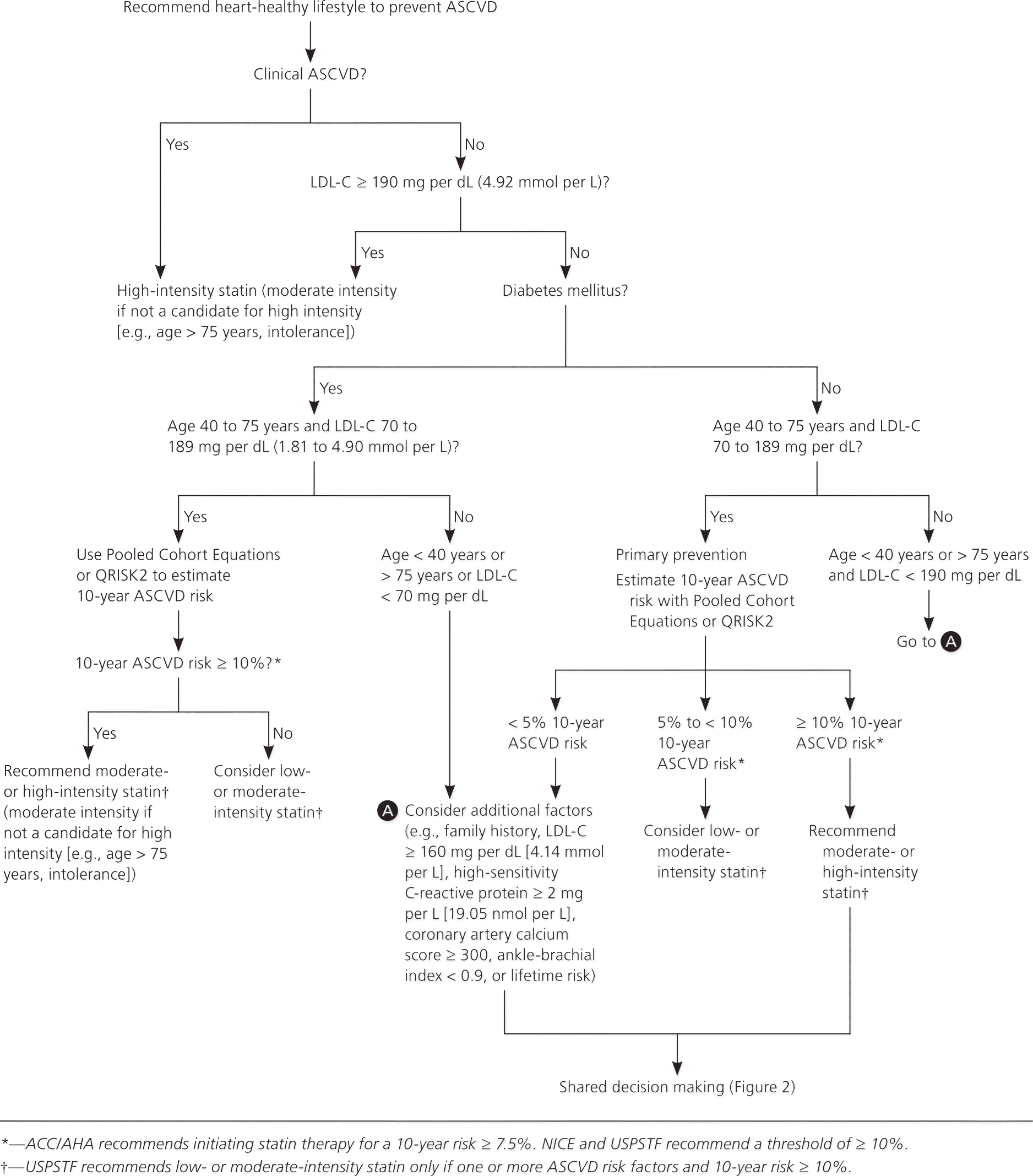
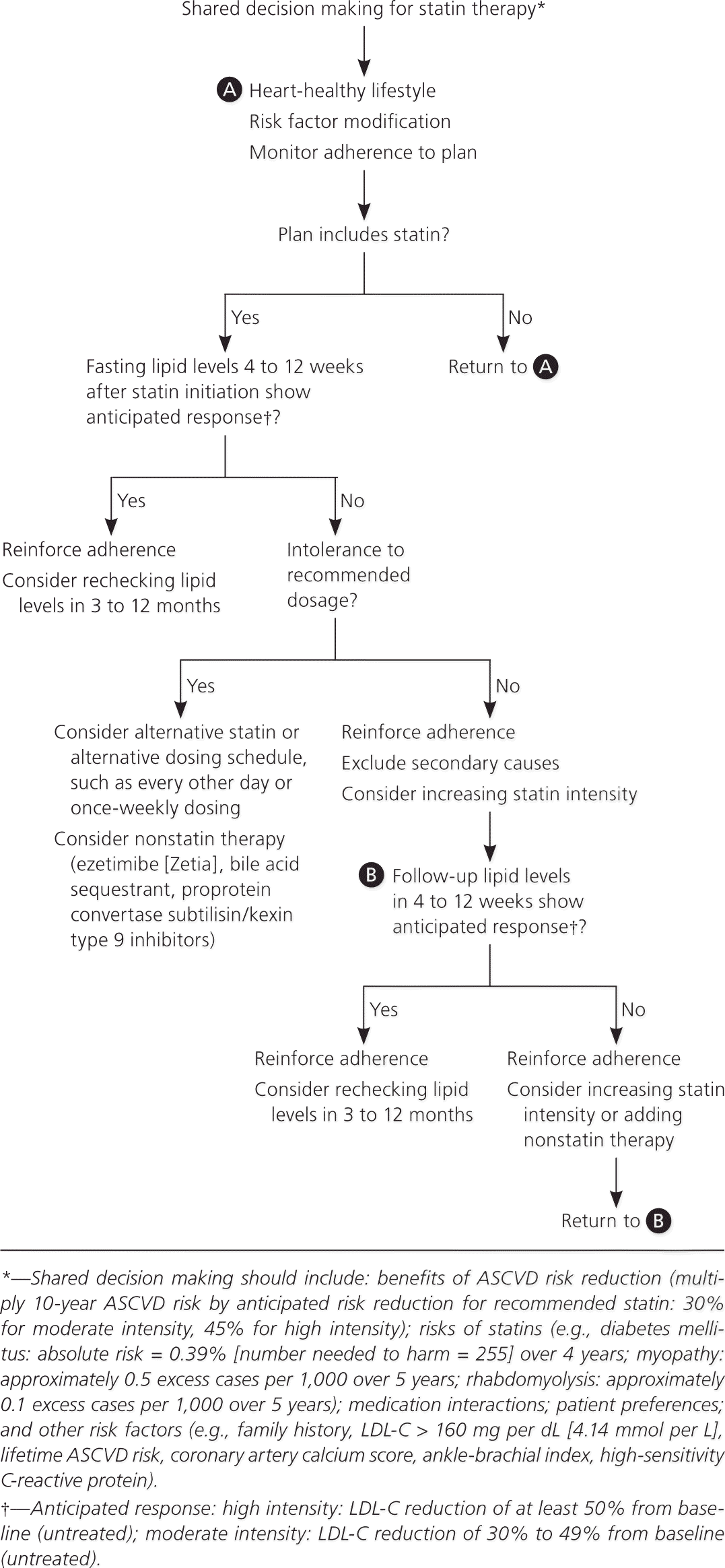
Although the ACC/AHA and NICE guidelines do not recommend treating to lipid goals, the ACC/AHA guideline recommends checking lipid levels four to 12 weeks after initiation of statins to determine a patient's adherence and every three to 12 months thereafter as clinically indicated. The NICE guideline recommends checking lipid levels three months after starting statins, but not routinely after that. Liver transaminase levels should be checked before starting statins. However, guidelines vary on if and when to recheck them in the absence of symptoms. Both guidelines emphasize that shared decision making is essential to any treatment plan (Figure 2).1,2,5
Risk Calculators
After reviewing validated risk-prediction tools, the ACC/AHA panel determined that none was sufficiently accurate for calculating the 10-year risk of an ASCVD event and subsequently developed the new Pooled Cohort Equations risk calculator that is available at http://www.cvriskcalculator.com. Concerns have been raised that the new calculator may overestimate risk by as much as 75% to 150%,6 but it is nonetheless more accurate for determining risk than the previously recommended Framingham risk calculator.7,8 The NICE guideline, however, recommends using the QRISK2 calculator, which is available at http://www.qrisk.org/.4 Clinicians should use the Pooled Cohort Equations risk calculator, the QRISK2 calculator, or both to determine a patient's risk.
Questions About the Guideline
Questions about the ACC/AHA guideline have focused on bias in the development. Nearly every panelist for the 2004 ACC/AHA guidelines, developed in collaboration with the National Heart, Lung, and Blood Institute,9 had industry ties. The ACC/AHA committee made efforts to exclude industry influence for the 2013 guideline, but seven of the 15 panelists still had ties to industry.10
Additionally, there is concern that current guidelines underestimate the risks associated with statins. Major risks from statin use include myopathy (incidence of 0.5 per 1,000 more than in the general population over five years) and rhabdomyolysis (incidence of 0.1 per 1,000 more than in the general population over five years).11 There is also a small increase in the risk of diabetes mellitus. Although the ACC/AHA guideline mentions a 0.1% risk of diabetes and some studies have found higher rates associated with statin use, a 2010 meta-analysis of 13 trials involving 91,140 patients found an overall absolute risk of diabetes of 0.39% (number needed to harm = 255) over four years.12
AAFP Endorsement
The American Academy of Family Physicians (AAFP) endorses the ACC/AHA guideline with qualifications13: The Pooled Cohort Equations risk calculator has not been validated and may overestimate risk. Using a cutoff of 7.5% for the 10-year risk of an ASCVD event rather than 10% significantly increases the number of individuals taking statins. Many of the ACC/AHA recommendations were based on expert opinion, and several members on the guideline panel had conflicts of interest. The AAFP has not commented on the NICE or USPSTF guidelines.
Primary Prevention of ASCVD
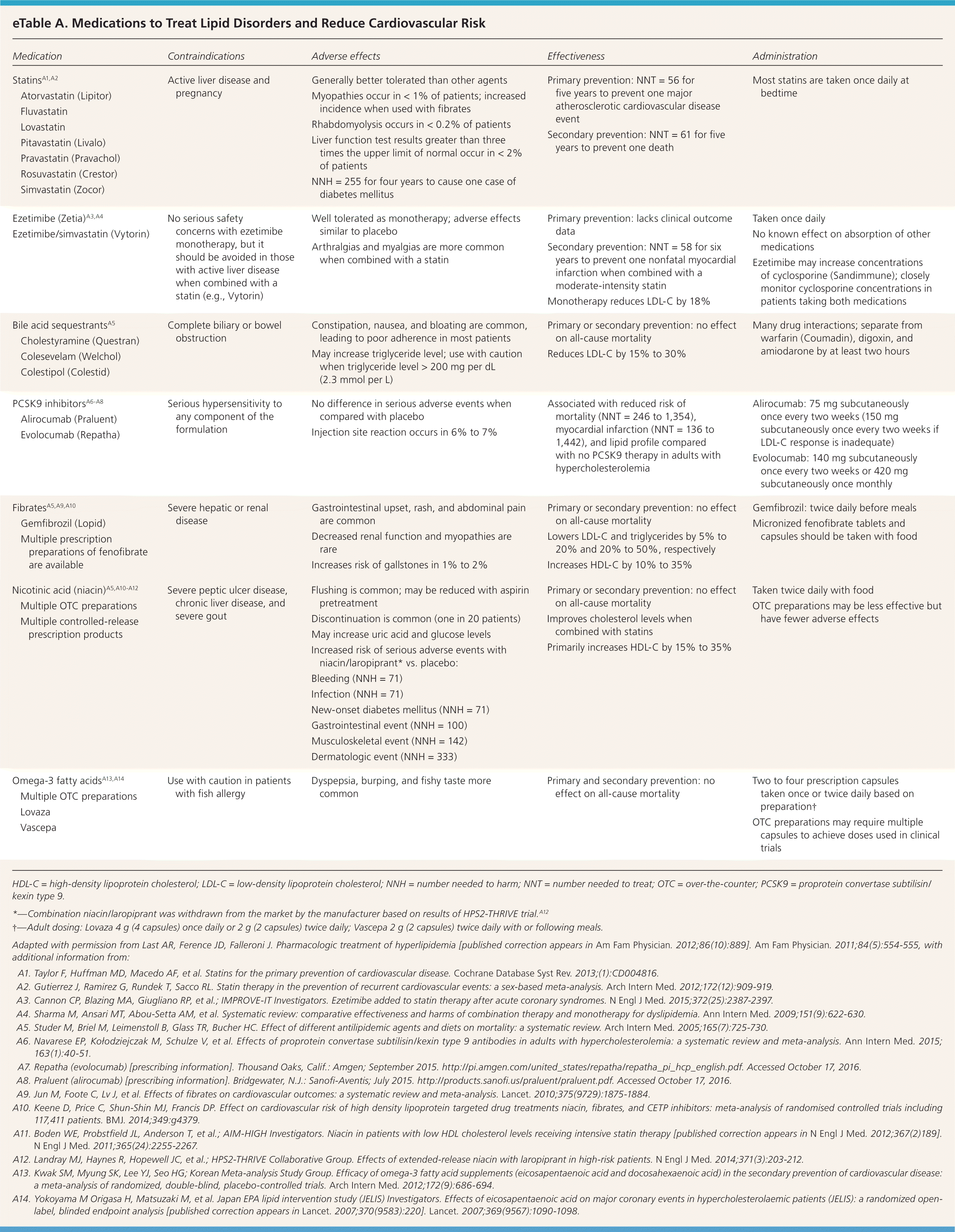
The foundation of primary prevention of ASCVD is therapeutic lifestyle modifications, which were reviewed in a previous issue of American Family Physician.14 To augment the benefits of therapeutic lifestyle modification, medications developed for the treatment of lipid disorders are sometimes used for primary prevention. eTable A summarizes the contraindications, adverse effects, effectiveness, and administration considerations for these medications.
| Medication | Contraindications | Adverse effects | Effectiveness | Administration | |
|---|---|---|---|---|---|
|
|
|
|
| |
|
|
|
| ||
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
STATINS
Statins have been shown to reduce the risk of ASCVD events when used for primary prevention, regardless of baseline risk. However, the magnitude of benefit becomes greater as the baseline risk of cardiovascular disease increases. For example, the five-year numbers needed to treat (NNT) for the prevention of one ASCVD event are 160, 108, 80, 54, and 40 for baseline 10-year ASCVD risks of 5%, 7.5%, 10%, 15%, and 20%, respectively.15 Similarly, when used for primary prevention, statins reduce overall mortality rates in patients with a baseline ASCVD risk of 10% or greater (five-year NNT = 250 to 500), but may not benefit those at lower risk (i.e., less than 10%).
Individuals with diabetes are at an increased lifetime risk of ASCVD events, and a high level of evidence supports the use of statin thrapy for the primary prevention of major ASCVD events in these patients 40 to 75 years of age.1 For individuals with diabetes who fall outside of this age range, statin therapy should be considered on an individual basis after considering risk reduction benefits, the potential for adverse effects and drug-drug interactions, and patient preferences.
A 2013 Cochrane review of 56,934 patients found that for every 1,000 persons treated with a statin for five years, 18 would avoid a major ASCVD event (i.e., myocardial infarction [MI], angina, or stroke; NNT = 56).11 Although the review focused on studies that included fewer than 10% of participants with known ASCVD, the inclusion of individuals with known cardiovascular disease may have overestimated the benefit of statins. A separate review indicated that statins are cost-effective for primary prevention.16
The Cholesterol Treatment Trialists' Collaboration provided further evidence in support of statin use for primary prevention of ASCVD events. The meta-analyses of 27 studies (n = 174,149), using individual participant data from the majority of statin trials, demonstrated a reduction in major ASCVD events (e.g., nonfatal MI, fatal coronary events, coronary revascularization, stroke) associated with statin use in low-risk individuals (five-year risk less than 10%). The authors found that for each reduction of 39 mg per dL (1.01 mmol per L) in LDL-C, there were 11 fewer major vascular events per 1,000 persons treated for five years.17 However, statin therapy for primary prevention was not associated with reduced rates of vascular death in patients with a five-year risk less than 10%. A secondary analysis evaluating all-cause mortality did not find a benefit from statin therapy in low-risk groups (i.e., 10-year risk less than 10%).18
Another recent trial comparing moderate-intensity statin therapy with placebo in patients at an intermediate risk of cardiovascular disease (i.e., approximately 1% annual cardiovascular event risk) also demonstrated a reduction in MI (NNT = 250), stroke (NNT = 200), coronary heart disease (NNT = 200), and cardiovascular-related hospitalization (NNT = 72) after a median of 5.6 years in patients receiving statins.19 Statin therapy was associated with an increased risk of cataract surgery (number needed to harm = 142) and muscle pain or weakness (number needed to harm = 91), and as with the Cholesterol Treatment Trialists' Collaboration, there were no differences in cardiovascular or all-cause mortality.
NONSTATINS
There is no convincing evidence that routine use of non-statin lipid-lowering medications (i.e., ezetimibe [Zetia], niacin, fibrates, and omega-3 fatty acids) are useful in the primary prevention of ASCVD.20 The addition of niacin demonstrated significant harm in a recent randomized controlled trial, and its use is no longer recommended.21 In early 2016, the U.S. Food and Drug Administration withdrew approvals for extended-release niacin and delayed-release fenofibric acid (fibrate) in combination with statins based on the lack of cardiovascular benefit reported in trials.22
In 2016, the ACC issued an Expert Consensus Decision Pathway (ECDP) on the role of nonstatin therapies in the management of ASCVD risk.5 The ECDP did not involve formal systematic reviews, grading of the evidence, or synthesis of the evidence. Rather, as expert opinion, its goal was to provide practical advice for clinicians and patients in situations not covered by the 2013 ACC/AHA guideline until more evidence emerges. The consensus statement recommends that nonstatin therapy should be considered in at-risk individuals who do not achieve the expected statin response (e.g., 50% or greater LDL-C reduction with high-intensity statin therapy or 30% to 49% LDL-C reduction with moderate-intensity statin therapy) or who cannot tolerate the recommended statin dose. Nonstatin therapies should not, however, be considered as alternatives to evidence-based statin therapy unless intolerance has been systematically determined. A decision support tool to assist clinicians in this process is available at http://www.acc.org/StatinIntoleranceApp.
If nonstatins are used because of documented statin intolerance or a failure to achieve an expected response with statins, the ECDP recommends ezetimibe as first-line therapy or bile acid sequestrants as second-line therapy (e.g., if a patient cannot tolerate ezetimibe and the triglyceride level is less than 300 mg per dL [3.4 mmol per L]) for primary prevention in patients with or without diabetes, a 10-year ASCVD risk of 10% or greater, and a baseline LDL-C level of 70 to 189 mg per dL (1.81 to 4.90 mmol per L).5 These recommendations also apply to patients without ASCVD and a baseline LDL-C level of 190 mg per dL (4.92 mmol per L) or greater. However, in these patients, it is reasonable to consider a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor in combination with maximally tolerated statin therapy before a bile acid sequestrant because PCSK9 inhibitors are more effective at lowering LDL-C levels. The ECDP recommends that complex cases be referred to lipid subspecialists.
As a new class of medications, PCSK9 inhibitors are showing early promise for the prevention of ASCVD. PCSK9 is a natural protein that binds to and destroys LDL receptors in the liver, thereby preventing them from removing LDL-C from the circulation. The PCSK9 inhibitors block this protein, allowing the receptors to continue clearing LDL-C. A 2015 meta-analysis of 24 trials involving adults with hypercholesterolemia (individual trials consisted of mixed populations of primary and secondary prevention) found significant reductions in MI (NNT = 136 to 1,442) and all-cause mortality (NNT = 246 to 1,354) in patients who received PCSK9 inhibitors combined with maximally tolerated statin therapy compared with patients who received placebo or ezetimibe.23
The studies in the meta-analysis were limited by their short duration of follow-up (two months to two years),23 and the use of these medications is limited by high cost and the need for administration by injection. Although promising, more data are needed on the long-term safety, effectiveness, and cost-effectiveness of PCSK9 inhibitors before they can be recommended for the primary prevention of ASCVD events in patients other than those with extreme LDL-C elevations (i.e., 190 mg per dL or greater).
Secondary Prevention of ASCVD
STATINS
Statins are recommended for secondary prevention in almost all patients with established ASCVD.1,2 Statins benefit patients with established ASCVD independently of sex or baseline cholesterol levels.24,25 In adults with a history of ASCVD, statins decrease the five-year risk of death (NNT = 61), MI (NNT = 47), and stroke (NNT = 189).26–28 A 2015 meta-analysis of 27 secondary-prevention studies comparing statins (or more intensive statin therapy) with a control group (or less intensive statin therapy) demonstrated similar reductions in major vascular events in men (NNT = 91 for five years) and in women (NNT = 143 for five years) for each 39-mg-per-dL reduction in LDL-C levels.25
The effectiveness of statin therapy for reducing cardiovascular risk does not appear to differ among statins and should be considered a class effect.29 The comparative effectiveness likely relates more to the intensity of dosing than to the specific agent used.
If high-intensity statins are indicated (Table 11–4 ) but the patient cannot tolerate this level of statins, moderate-intensity statin therapy is the preferred approach. Similarly, moderate-intensity statins can be used in patients with established ASCVD who have a risk factor for statin-induced adverse effects, such as advanced age, frailty or small body frame, hypothyroidism, surgery (i.e., during the perioperative period), or alcohol abuse.30 In cases of true statin intolerance (i.e., intolerance to two or three statins with at least one at the lowest dose), nonstatin therapies may be considered.
NONSTATINS
To date, there are no convincing clinical data showing that niacin, fibrates, or omega-3 fatty acids, either as monotherapy or in addition to high-intensity statins, provide additional benefit for secondary prevention of ASCVD events with an acceptable margin of safety beyond that of high-intensity statins.1,31–40
However, there is some initial evidence that ezetimibe may have a role in secondary prevention of ASCVD. The IMPROVE-IT trial randomized patients who experienced a recent acute coronary syndrome event to ezetimibe plus moderate-intensity simvastatin (Vytorin; 40 mg daily) or to placebo plus simvastatin (Zocor; 40 mg daily).31 Combination therapy produced a 2% absolute risk reduction of the composite outcome (cardiovascular death, MI, or nonfatal stroke; 32.7% vs. 34.7%; NNT = 50 for six years). Most of the benefit came from a reduction in nonfatal MIs (NNT = 58 over six years). All-cause mortality, cardiovascular death, and discontinuation of medication because of adverse events did not differ between groups. It is important to note that the comparison group received less than the recommended dose of a statin (i.e., moderate-intensity rather than high-intensity). The most conservative conclusion that can be drawn from this trial is that a moderate-intensity statin plus ezetimibe may serve as an alternative in patients with acute coronary syndrome who do not tolerate high-intensity statin therapy.
The ACC's ECDP acknowledges a gap in evidence from randomized controlled trials demonstrating benefit when adding nonstatins to statin therapy in patients with stable clinical ASCVD (i.e., an ASCVD event more than three months prior) who have not achieved an expected response to a statin. Ezetimibe (first line), a bile acid sequestrant (second line, if intolerant to ezetimibe and triglyceride level is less than 300 mg per dL), or a PCSK9 inhibitor (added to maximally tolerated statin and either ezetimibe or bile acid sequestrant) may be reasonable if patients and clinicians agree after engaging in shared decision making.5 The discussion should address the potential for further ASCVD risk reduction that can be expected from adding a nonstatin, the potential for adverse events or drug-drug interactions, and patient preferences. However, intensifying lifestyle modifications and addressing other major ASCVD risk factors should be considered before adding nonstatin therapy.
In patients with documented statin intolerance, the ECDP recommends referral to a lipid subspecialist. Ezetimibe, a bile acid sequestrant, or both are recommended in these patients. A PCSK9 may be considered only if use of these medications does not produce a greater than 50% reduction in LDL-C levels.
Statins for Specific Vascular Conditions
ACUTE CORONARY SYNDROME
High-intensity statin therapy is recommended for acute coronary syndrome. A systematic review of three randomized trials comparing high-intensity vs. moderate-intensity statins in patients with acute coronary syndrome demonstrated reductions in all-cause mortality (NNT = 85; 95% confidence interval [CI], 55 to 236) and cardiovascular mortality (NNT = 109; 95% CI, 69 to 469) with high-intensity statin therapy.27
CEREBROVASCULAR DISEASE
Statin use lowers the risk of stroke, even in moderate-risk individuals (five-year ASCVD risk of 10% or less), with an NNT of 90 over five years, independent of age, sex, LDL-C levels, or previous diagnosis of vascular disease.17 There was concern that statins might increase the risk of hemorrhagic stroke and offset the benefits, but a meta-analysis of 31 trials did not demonstrate an association between statin use and hemorrhagic stroke risk.41
PERIPHERAL ARTERIAL DISEASE AND AORTIC ANEURYSM
A Cochrane review found that statin use among patients with peripheral arterial disease nearly doubled total walking distance, from an average of 175 to 327 m (574 to 1,073 ft); it also increased pain-free walking distances from 148 to 238 m (486 to 781 ft).42 A 2014 meta-analysis revealed that in patients with peripheral arterial disease, statin use reduced all-cause mortality (NNT = 12; 95% CI, 9 to 23).43
Similarly, statin use in patients with an abdominal aortic aneurysm repair lowers mortality rates (NNT = 12 for five years; 95% CI, 8 to 25).44 There is not, however, an effect on the rate of aneurysm expansion in patients who have not undergone repair.
Statin Therapy in Specific Populations
OLDER ADULTS
Few clinical trials have evaluated the use of statins in older patients. In a 2013 meta-analysis of eight trials that included patients with a mean age of 73.0 ± 2.9 years who did not have clinical ASCVD, statin use was associated with a reduced risk of MI (NNT = 45 to 171) and stroke (NNT = 97 to 511).45 There was no difference, however, in all-cause mortality.
An earlier meta-analysis found that in patients 62 to 85 years of age with ASCVD, statin use reduced the risk of death (NNT = 28; 95% CI, 15 to 56), nonfatal MI (NNT = 38; 95% CI, 16 to 118), and stroke (NNT = 58; 95% CI, 27 to 177) over five years.46
Frail patients may be more likely to have adverse effects with statin therapy. Despite this, frail older adults still benefit; just one year of statin therapy has been demonstrated to lower mortality rates (adjusted hazard ratio = 0.67; 95% CI, 0.53 to 0.84) and slow physical functional decline.47
The NICE guideline recommends treating older patients up to 84 years of age the same as adults of any age, and that moderate-intensity statins should be considered for the prevention of nonfatal MI in patients older than 85 years.2 The USPSTF guideline concludes there is insufficient evidence to balance the risks and benefits of primary prevention with statins in persons 76 years and older.3 The ACC/AHA guideline does not have recommendations for patients older than 75 years unless they have clinical ASCVD, recommending moderate-intensity rather than high-intensity statins in those patients.1 Shared decision making is especially important in frail older adults because the risk of adverse effects increases in this population while life expectancy is diminished, which may offset the potential benefits.
CHRONIC KIDNEY DISEASE
In patients with chronic kidney disease who do not require dialysis, statin therapy reduces the risk of major cardiovascular events (NNT = 16 to 25), all-cause mortality (NNT = 36 to 124), and cardiovascular mortality (NNT = 56 to 116).48 However, statin use may not reduce all-cause or cardiovascular mortality in patients on dialysis.49
For patients with chronic kidney disease, there is evidence to support the addition of ezetimibe to statin therapy. The SHARP trial found reductions in ischemic strokes (NNT = 112) and coronary revascularization (NNT = 84) with ezetimibe/simvastatin compared with placebo over five years in patients with chronic kidney disease.50 Moderate- to high-intensity statin therapy should be recommended for primary prevention in patients with stage 3b or more severe chronic kidney disease.
PREGNANCY
Because of the risks of fetal harm, women taking statins should be advised to discontinue treatment immediately if they become pregnant, and they should be counseled on intensive lifestyle modifications.5 Bile acid sequestrants may be appropriate in the interim. Statin therapy may be resumed after completion of breastfeeding. Women who plan to become pregnant should discontinue statin use at least one to three months before attempting to conceive.
This article updates previous articles on this topic by Last, et al.,51 and Safeer and Lacivita.52
Data Sources: A PubMed search was completed in Clinical Queries using the key terms lipid, cholesterol, prevention, pharmacotherapy, treatment, ASCVD and subtypes CHD, stroke, PAD, as well as for the specific treatments considered (e.g., statins, fibrates). The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. The following databases and summaries were also used: Cochrane Database of Systematic Reviews, BMJ Clinical Evidence, National Guideline Clearinghouse, and Essential Evidence Plus. Search dates: July 2015 through October 2016.