Am Fam Physician. 2018;97(2):102-110
Author disclosure: No relevant financial affiliations.
Noninfectious penile lesions are classified by clinical presentation as papulosquamous (e.g., psoriasis), inflammatory (e.g., lichen sclerosus, lichen nitidus, lichen planus), vascular (e.g., angiokeratomas), or neoplastic (e.g., carcinoma in situ, invasive squamous cell carcinoma). Psoriasis presents as red or salmon-colored plaques with overlying silvery scales, often with extragenital cutaneous lesions. Lichen sclerosus presents as a phimotic, hypopigmented prepuce or glans penis with a cellophane-like texture. Lichen nitidus usually produces asymptomatic pinhead-sized, hypopigmented papules. The lesions of lichen planus are pruritic, violaceous, polygonal papules that are typically systemic. Angiokeratomas are typically asymptomatic, well-circumscribed, red or blue papules, often with annular or figurate configurations. Carcinoma in situ should be suspected if there are velvety red or keratotic plaques on the glans penis or prepuce, whereas invasive squamous cell carcinoma presents as a painless lump, ulcer, or fungating mass. Some benign lesions, such as psoriasis and lichen planus, may mimic carcinoma in situ or invasive squamous cell carcinoma. Biopsy is indicated if the diagnosis is in doubt or neoplasm cannot be excluded. The management of benign noninfectious penile lesions usually involves observation, topical corticosteroids, or topical calcineurin inhibitors. Neoplastic lesions generally warrant organ-sparing surgery.
Diagnosis and management of cutaneous penile lesions can be challenging because of lack of physician familiarity and patient embarrassment. Even though noninfectious lesions are common, penile lesions are often attributed to infectious causes, especially in younger patients.1 The key to efficient diagnosis is a genitourinary examination that defines the predominant characteristic of the lesions2,3 (Table 13). Noninfectious penile lesions are classified by clinical presentation as papulosquamous (e.g., psoriasis), inflammatory (e.g., lichen sclerosus, lichen nitidus, lichen planus), vascular (e.g., angiokeratomas), or neoplastic (e.g., carcinoma in situ, invasive squamous cell carcinoma). Lesions localized to the penis usually involve different diagnostic and treatment considerations than those with extragenital findings. Biopsy is typically reserved for an unclear diagnosis, or if neoplasm cannot be excluded. Management options for noninfectious penile lesions are summarized in Table 2.3
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Topical corticosteroids are first-line therapy for psoriasis and lichen sclerosus. | A | 4, 8, 11, 14, 15, 29, 34, 35 | Psoriasis: consistent findings from multiple observational studies Lichen sclerosus: meta-analysis, consensus guidelines |
| Observation is sufficient for lichen nitidus and angiokeratomas because most cases are asymptomatic. | C | 38, 41, 52, 54, 55 | Observational studies |
| Topical corticosteroids are first-line therapy for lichen planus. | C | 1, 44, 46 | Observational studies |
| Circumcision is first-line therapy for isolated preputial carcinoma in situ. | C | 70, 71 | Observational studies and guidelines |
| Biopsy should be performed on any lesion for which penile carcinoma in situ or penile cancer cannot be excluded. | C | 66 | Expert opinion |
| Circumcision is the first-line therapy for isolated preputial invasive squamous cell carcinoma. | C | 66, 78, 79 | Observational studies and guidelines |
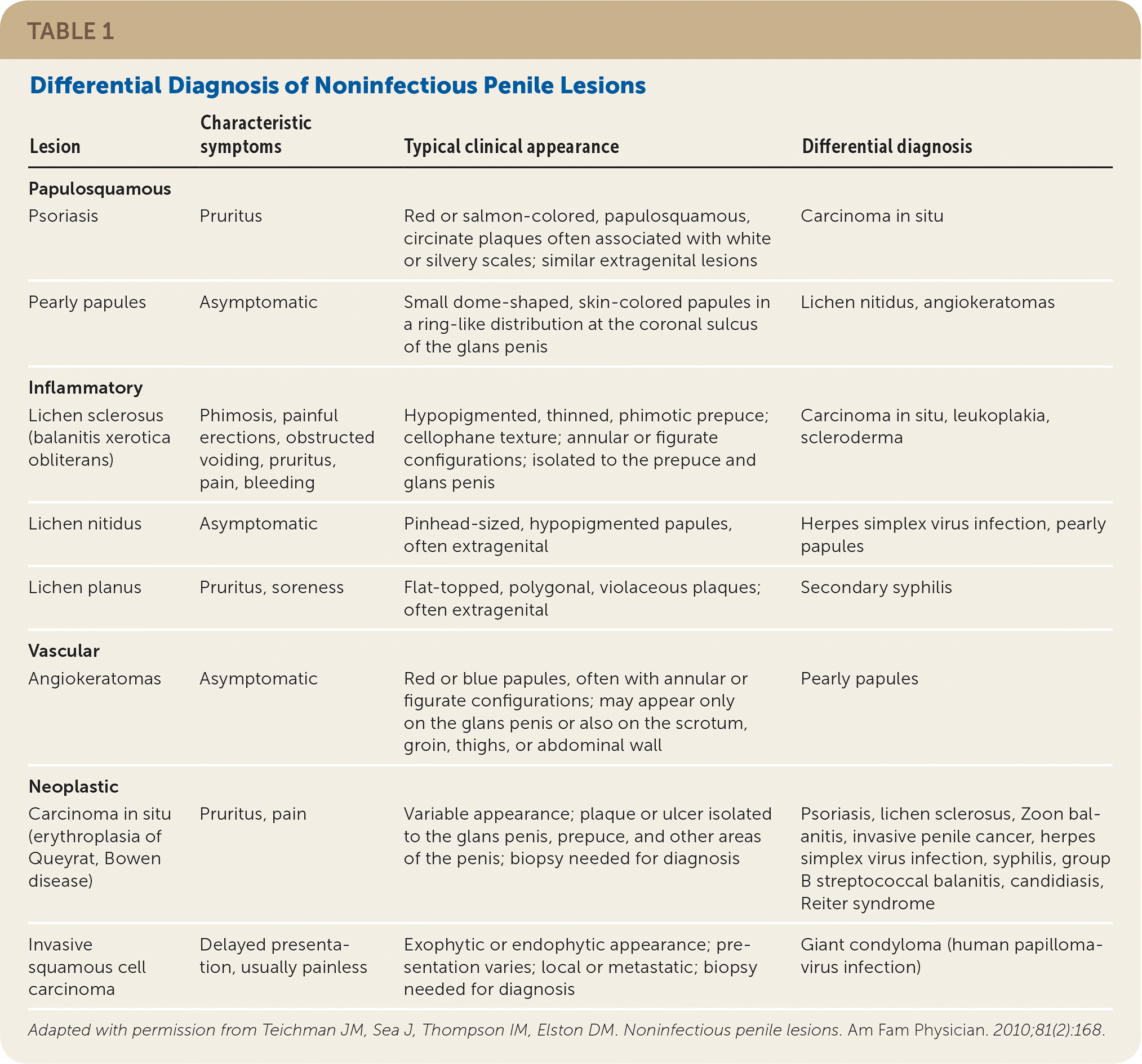
| Lesion | Characteristic symptoms | Typical clinical appearance | Differential diagnosis |
|---|---|---|---|
| Papulosquamous | |||
| Psoriasis | Pruritus | Red or salmon-colored, papulosquamous, circinate plaques often associated with white or silvery scales; similar extragenital lesions | Carcinoma in situ |
| Pearly papules | Asymptomatic | Small dome-shaped, skin-colored papules in a ring-like distribution at the coronal sulcus of the glans penis | Lichen nitidus, angiokeratomas |
| Inflammatory | |||
| Lichen sclerosus (balanitis xerotica obliterans) | Phimosis, painful erections, obstructed voiding, pruritus, pain, bleeding | Hypopigmented, thinned, phimotic prepuce; cellophane texture; annular or figurate configurations; isolated to the prepuce and glans penis | Carcinoma in situ, leukoplakia, scleroderma |
| Lichen nitidus | Asymptomatic | Pinhead-sized, hypopigmented papules, often extragenital | Herpes simplex virus infection, pearly papules |
| Lichen planus | Pruritus, soreness | Flat-topped, polygonal, violaceous plaques; often extragenital | Secondary syphilis |
| Vascular | |||
| Angiokeratomas | Asymptomatic | Red or blue papules, often with annular or figurate configurations; may appear only on the glans penis or also on the scrotum, groin, thighs, or abdominal wall | Pearly papules |
| Neoplastic | |||
| Carcinoma in situ (erythroplasia of Queyrat, Bowen disease) | Pruritus, pain | Variable appearance; plaque or ulcer isolated to the glans penis, prepuce, and other areas of the penis; biopsy needed for diagnosis | Psoriasis, lichen sclerosus, Zoon balanitis, invasive penile cancer, herpes simplex virus infection, syphilis, group B streptococcal balanitis, candidiasis, Reiter syndrome |
| Invasive squamous cell carcinoma | Delayed presentation, usually painless | Exophytic or endophytic appearance; presentation varies; local or metastatic; biopsy needed for diagnosis | Giant condyloma (human papillomavirus infection) |
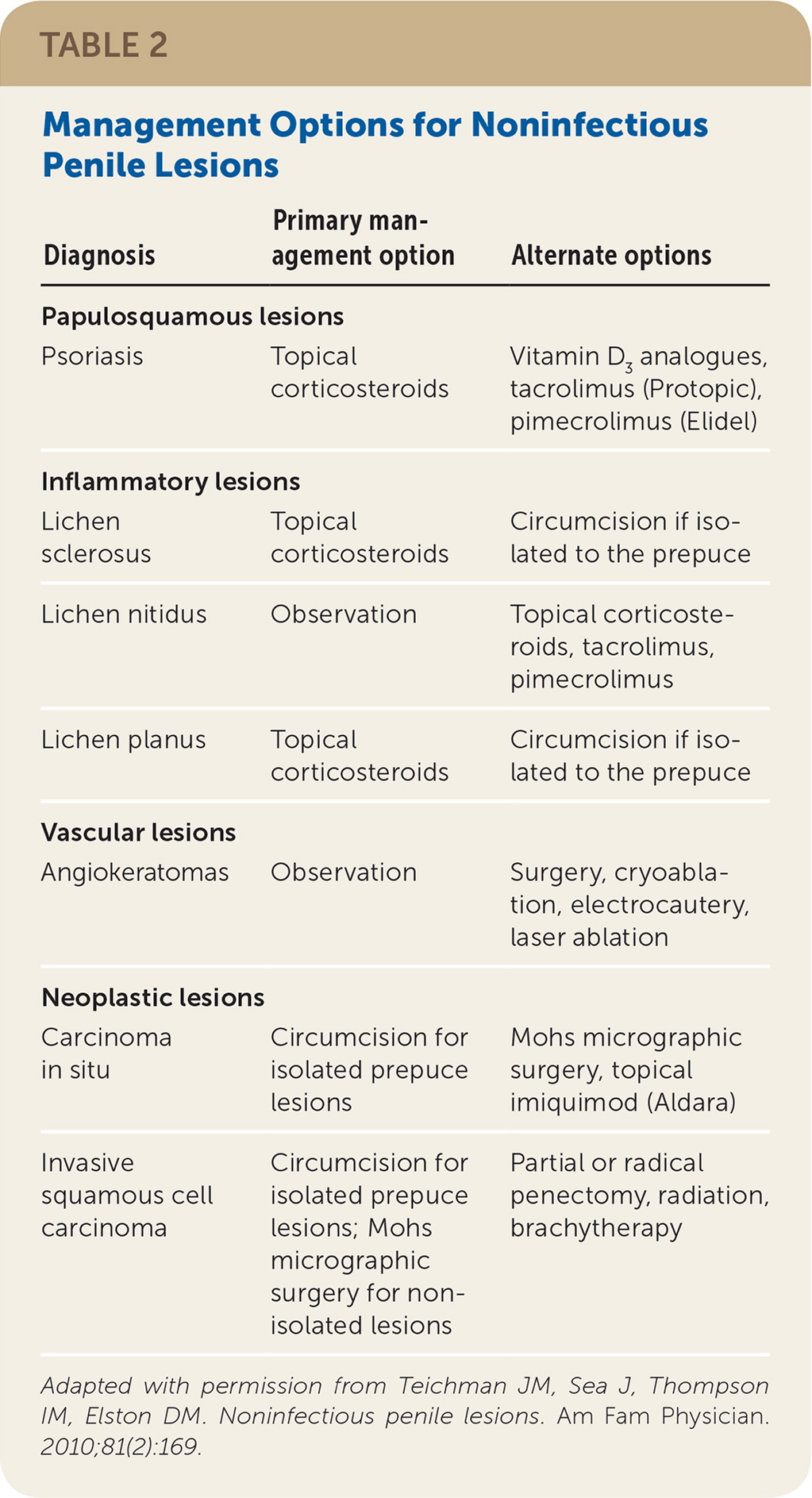
| Diagnosis | Primary management option | Alternate options |
|---|---|---|
| Papulosquamous lesions | ||
| Psoriasis | Topical corticosteroids | Vitamin D3 analogues, tacrolimus (Protopic), pimecrolimus (Elidel) |
| Inflammatory lesions | ||
| Lichen sclerosus | Topical corticosteroids | Circumcision if isolated to the prepuce |
| Lichen nitidus | Observation | Topical corticosteroids, tacrolimus, pimecrolimus |
| Lichen planus | Topical corticosteroids | Circumcision if isolated to the prepuce |
| Vascular lesions | ||
| Angiokeratomas | Observation | Surgery, cryoablation, electrocautery, laser ablation |
| Neoplastic lesions | ||
| Carcinoma in situ | Circumcision for isolated prepuce lesions | Mohs micrographic surgery, topical imiquimod (Aldara) |
| Invasive squamous cell carcinoma | Circumcision for isolated prepuce lesions; Mohs micrographic surgery for non-isolated lesions | Partial or radical penectomy, radiation, brachytherapy |
Papulosquamous Lesions
PSORIASIS
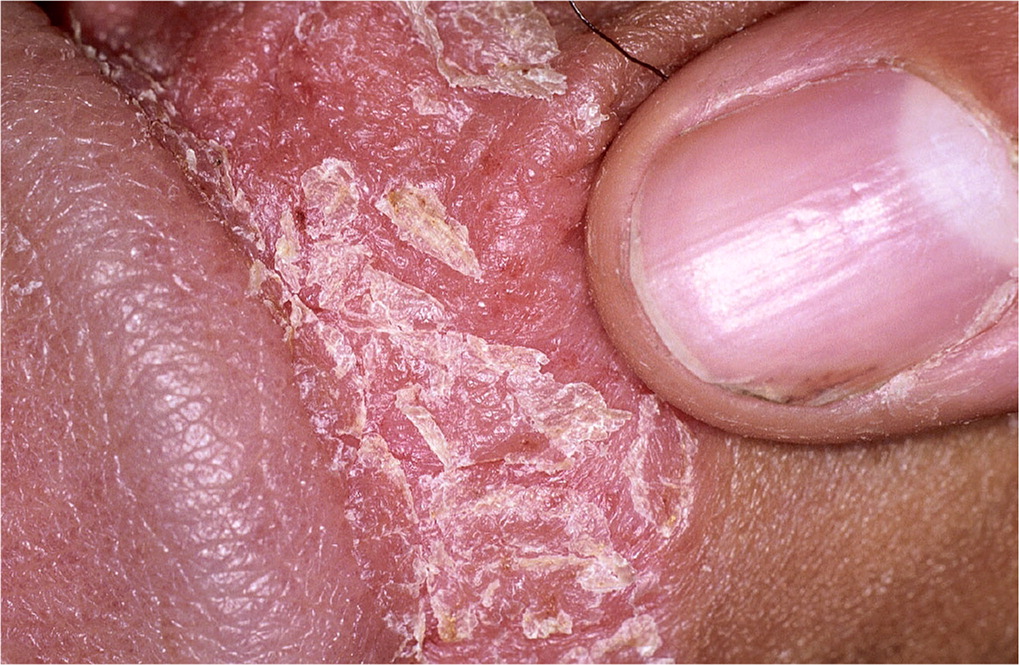
Psoriasis may occur at any age, with bimodal peaks at 16 to 22 years and 57 to 60 years.4,5 The prevalence of psoriasis in the United States is 1% to 2%, with genital involvement occurring in up to 40% of patients.5–9 Psoriasis typically presents as red or salmon-colored, papulosquamous, circinate plaques often associated with white or silvery scales4,7,10 (Figure 13). Exacerbating factors include stress, excessive alcohol and tobacco use, acute infections (particularly streptococcal infections), and some medications (e.g., propranolol, lithium).11 Extragenital psoriasis occurs on extensor surfaces and aids diagnosis.12,13
Psoriatic treatment depends on whether the disease is localized or disseminated.5,7,9,14 First-line localized treatment includes once-daily application of low- to moderate-potency topical corticosteroids.4,8,11,14 As a general rule, no more than 50 mg of ultra-high-potency (group 1) or 100 mg of high-potency (group 2) topical corticosteroids should be applied over a long-term period because continuous use may cause skin atrophy 14 (Table 3). Lesions may reappear when corticosteroid use is discontinued.15 In the rare case that an ultra-high-potency topical corticosteroid is indicated on an ongoing basis, use should be limited to weekend pulse dosing (once-daily application two times per week).16,17 Intermittent use of topical corticosteroids as maintenance therapy (once or twice per week) on areas that commonly flare is recommended to help prevent relapses and is more effective than use of emollients alone.16 Oral and topical vitamin D analogues provide comparable effectiveness to corticosteroid monotherapy.18 If long-term therapy is required, calcineurin inhibitors such as tacrolimus (Protopic) or pimecrolimus (Elidel) may be appropriate and could aid in preventing atrophy, but they may also cause pruritus.19,20
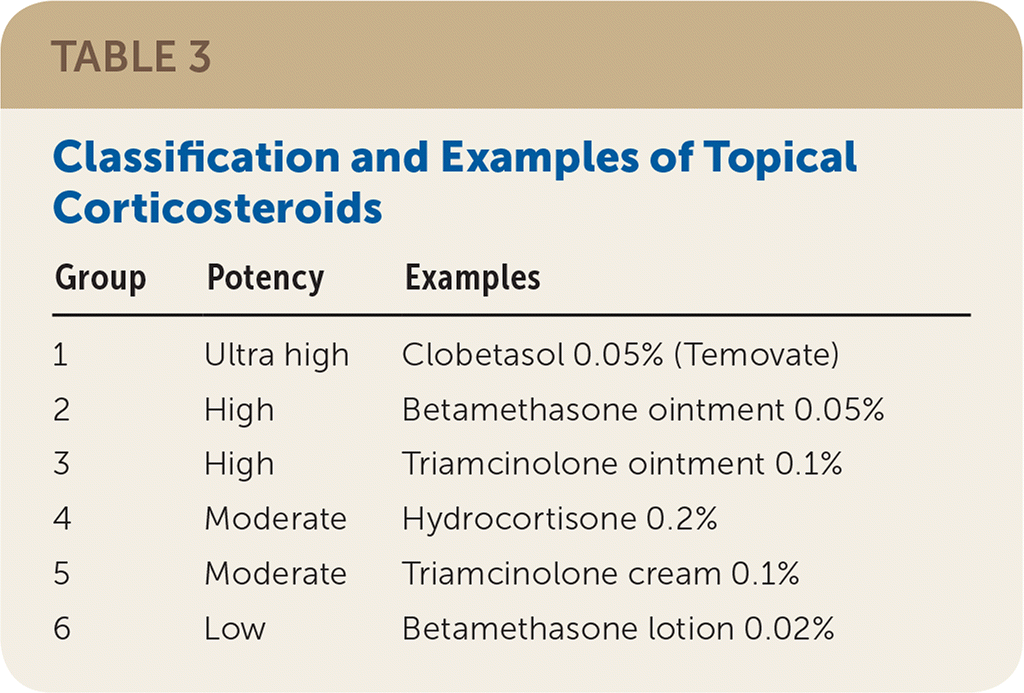
| Group | Potency | Examples |
|---|---|---|
| 1 | Ultra high | Clobetasol 0.05% (Temovate) |
| 2 | High | Betamethasone ointment 0.05% |
| 3 | High | Triamcinolone ointment 0.1% |
| 4 | Moderate | Hydrocortisone 0.2% |
| 5 | Moderate | Triamcinolone cream 0.1% |
| 6 | Low | Betamethasone lotion 0.02% |
Biologic therapy is expensive, but it may be appropriate for refractory or widespread disease or in patients with psoriatic arthritis. Lymphocyte function–associated antigen-1, interleukin-12, interleukin-23, tumor necrosis factor, and T cell activation have been identified as biologic targets in psoriatic treatment.21 Several biologics are available to treat psoriasis, including etanercept (Enbrel), infliximab (Remicade), and ustekinumab (Stelara).22 Despite limited clinical use, there is emerging evidence for combining these newer biologics with more traditional therapies for severe resistant disease, such as methotrexate, cyclosporine (Sandimmune), acitretin (Soriatane), and phototherapy.22
Inflammatory Lesions
LICHEN SCLEROSUS
Penile lichen sclerosus, also known as balanitis xerotica obliterans, can occur at any age.23 The average age of patients at diagnosis is 42 years, and the prevalence is one in 300 males.24–28 Lichen sclerosus is associated with squamous cell carcinoma in 4% to 6% of patients.8,25,29 Genital lichen sclerosus is considered a precancerous condition.23,27,30
Lichen sclerosus appears as a hypopigmented lesion with a skin texture similar to crinkled paper or cellophane. It primarily affects the glans penis and prepuce (Figure 23). Bullae, erosions, or atrophy may be prominent. Patients typically present with phimosis, painful erections, obstructed voiding, pruritus, pain, and bleeding.27,31 Because lesions may cause obstruction of the urethra, urinary retention may be the initial presenting concern.27,29,32,33 Although lichen sclerosus can affect almost all parts of the body, some persons may be asymptomatic.23,27,32 Lichen sclerosus should be differentiated from carcinoma in situ (Figure 33), leukoplakia, and scleroderma.27,32 Biopsy is indicated if squamous cell carcinoma cannot be excluded.31
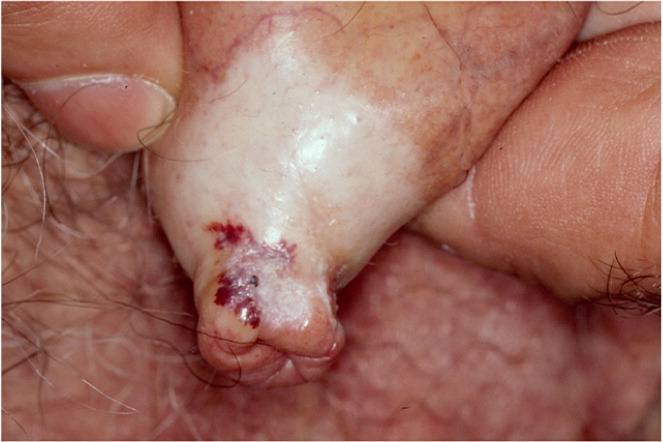
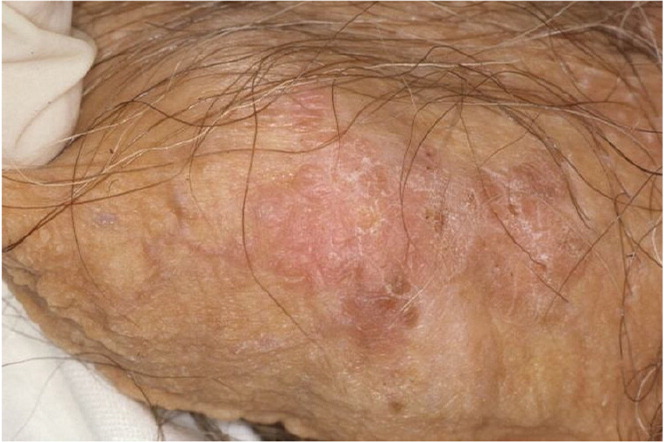
Lichen sclerosus is generally treated with moderate- to ultra-high-potency fluorinated topical corticosteroids to reduce symptoms and prevent malignant transformation.24,29,34,35 Weekend pulse therapy with an ultra-high-potency corticosteroid is effective and safer than long-term daily use of a less potent corticosteroid. Tacrolimus or pimecrolimus may also be effective, but long-term safety has not been established.29,35,36 Surgery is indicated for persistent disease or if there is clinical suspicion that the penile lesions might be squamous cell carcinoma.29 Circumcision may be indicated in uncircumcised patients with lichen sclerosus limited to the glans penis and prepuce. Severe cases may require reconstructive surgery, although conservative management may be appropriate if the risks of surgery outweigh the potential benefits.27,31,37 Systemic agents, such as retinoids and methotrexate, are reserved for severe cases of lichen sclerosus and when local therapy is ineffective.30,31 Long-term follow-up with periodic physical examination is appropriate to monitor for malignant transformation.28,29
LICHEN NITIDUS
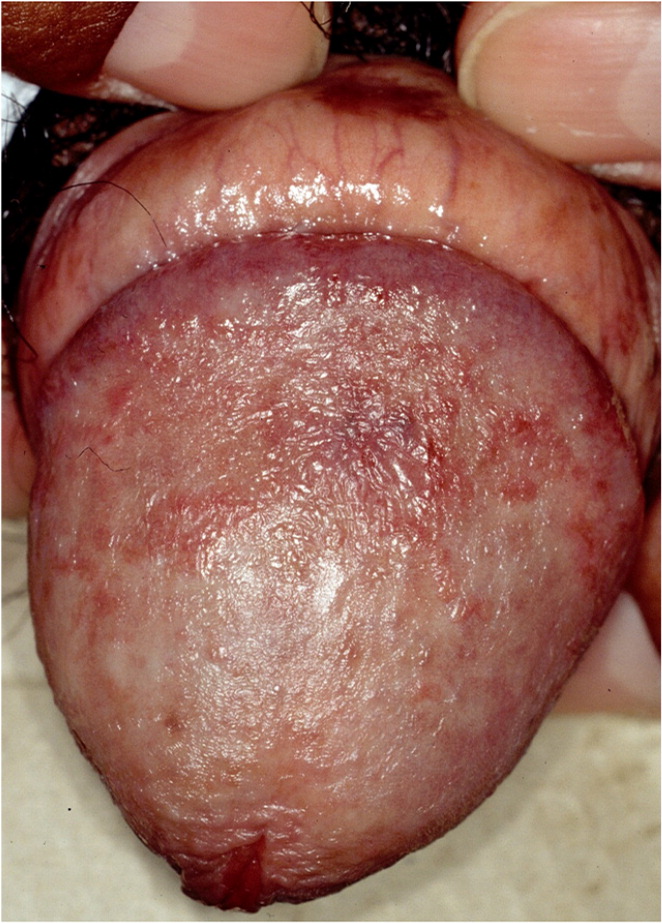
Lichen nitidus is uncommon. Patients typically present with discrete, slightly elevated, hypopigmented 1-mm papules on the penis, upper extremities, and abdomen38–41 (Figure 4A3). These lesions should be distinguished from pearly papules, which have a ring-like distribution on the coronal sulcus (Figure 4B). Patients are usually asymptomatic and do not require treatment, and lesions may resolve spontaneously.38,40,41 When treatment is indicated for cosmesis, options include corticosteroids and topical calcineurin inhibitors.40,42
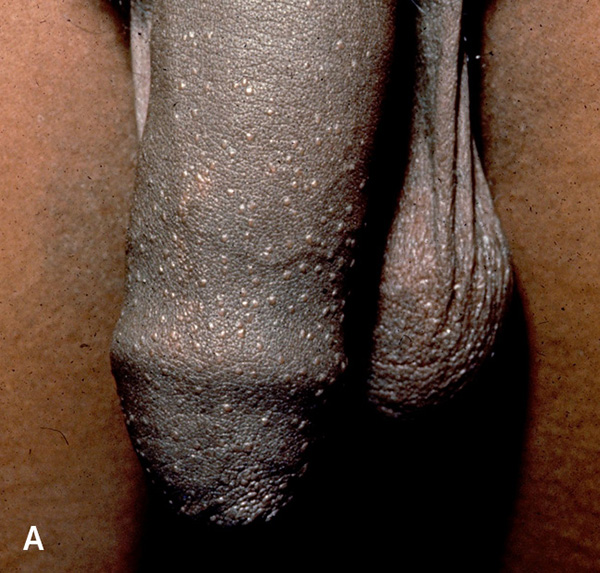
LICHEN PLANUS
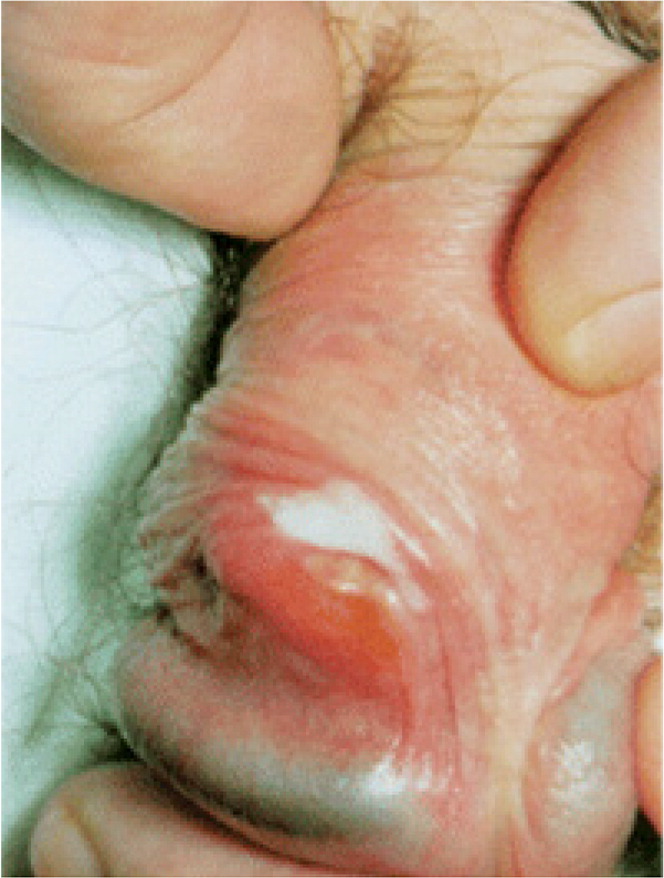
Lichen planus is typically systemic, affecting mucous membranes, nails, acral sites, and the scalp.43 One-fourth of patients with lichen planus have genital lesions, and most patients have extragenital involvement.44,45 Lichen planus lesions are raised, violaceous, flat-topped, leukokeratosis-like, polygonal papules46 (Figure 5A). Fine white streaks (Wickham striae) may appear on the surface of the lesions. In uncircumcised patients, the lesions assume a lacy, white, reticulated pattern1 (Figure 5B3). Patients with lichen planus often report ulcerated lesions and concerns of pruritus and soreness.43 Ulcerated or indurated lichen planus lesions (Figure 5C) suggest squamous cell carcinoma (eFigure A) and require biopsy.47
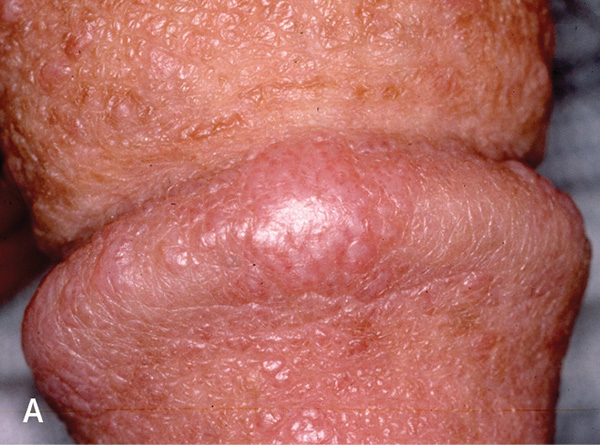
Treatment of lichen planus with weekend dosing of ultra-high-potency topical corticosteroids may be an effective strategy that carries less risk of atrophy than does daily dosing.16,17 Topical calcineurin inhibitors have been shown to be effective.48 For isolated lichen planus of the prepuce, circumcision is indicated if medical management is ineffective, although the disease may koebnerize (i.e., localize in areas of injury).49
Vascular Lesions
ANGIOKERATOMAS
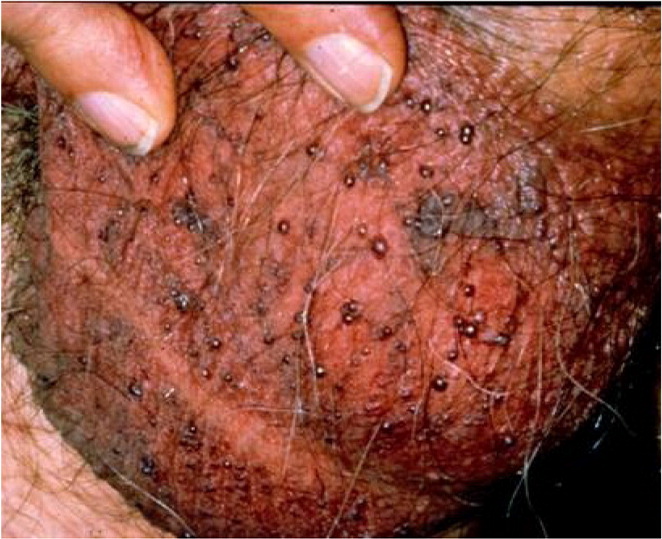
Angiokeratomas, which have a prevalence of less than 1%, are benign, well-circumscribed, red or blue papules of 1 to 6 mm that typically occur in patients older than 40 years.50–54 Diagnosis is usually made by characteristic appearance, although it may be misdiagnosed as penile cancer or pearly papules. Angiokeratomas may affect only the glans penis, scrotum, groin, thighs, or abdominal wall55 (Figure 6 and eFigure B). Patients with angiokeratomas may experience rare intermittent bleeding, pain, or pruritus.56 Angiokeratomas affecting the penile shaft, suprapubic region, or sacrum are associated with Fabry disease (angiokeratomas, anhidrosis, renal failure, hypertension, cardiomyopathy, neuropathic pain) and should prompt referral.57 Angiokeratomas are usually asymptomatic, so treatment is not required.53,55,56 However, treatment is indicated if the patient is symptomatic or if the lesions bleed. Options include surgery, cryoablation, electrocautery, and laser ablation.58–61
Neoplastic Lesions
CARCINOMA IN SITU
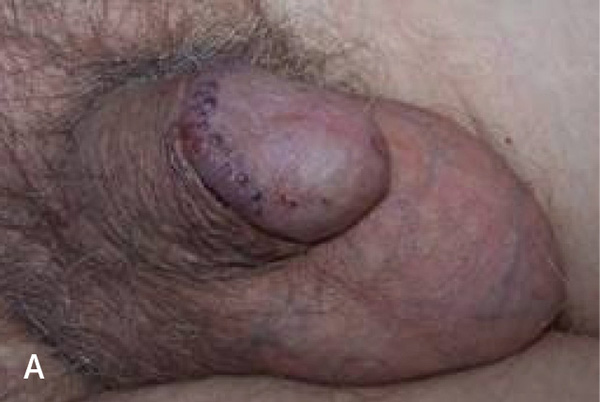
Penile carcinoma in situ is a premalignant lesion that typically affects uncircumcised men older than 60 years. Velvety plaques of the glans penis are known as erythroplasia of Queyrat; keratotic plaques are known as Bowen disease and can occur on the penile shaft, scrotal skin, or perineum8,31,62 (Figure 73 and eFigure C). Human papillomavirus is the primary etiology of penile carcinoma in situ, although other factors may include smegma and trauma from friction, heat, or inflammation.63,64 Penile carcinoma in situ progresses to invasive squamous cell carcinoma in approximately 5% to 30% of patients.65,66
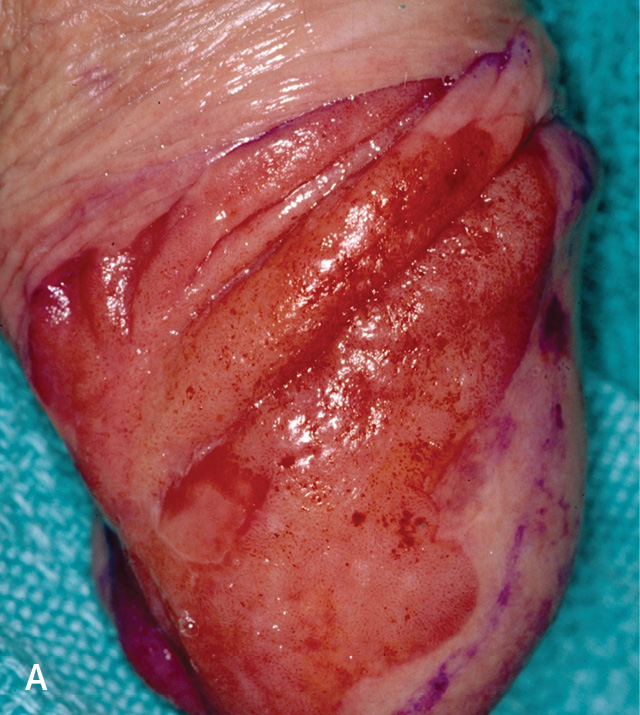
Pruritus and pain occur in approximately 50% of patients with penile carcinoma in situ.65 Lesions usually appear as raised, beefy red, velvety, irregularly shaped plaques that may ulcerate (eFigure A). The lesions are generally 2 to 35 mm and occur on the glans penis, urethral meatus, frenulum, coronal sulcus, or prepuce. In uncircumcised patients, the lesions may be crusted without a velvety red appearance. Lesions on the shaft may appear erythematous, display fissuring, and have soft, white scales. Penile carcinoma in situ requires biopsy to distinguish it from psoriasis and Zoon balanitis.8,67–69
Penile carcinoma in situ lesions restricted to the prepuce are treated with circumcision.70,71 Mohs micrographic surgery may be indicated for recurrence or incompletely excised lesions.63,71 Treatment with fluorouracil, curettage, local excision, laser ablation, or photodynamic therapy is associated with significant recurrence rates and requires thorough follow-up (repeat physical examinations and rebiopsy as clinically suspected).62,71 Radiation may be an option for patients who are not surgical candidates or who refuse surgery. Imiquimod is an immune response modifier that has also been studied for penile carcinoma in situ with mixed results.63,64,72,73
INVASIVE SQUAMOUS CELL CARCINOMA
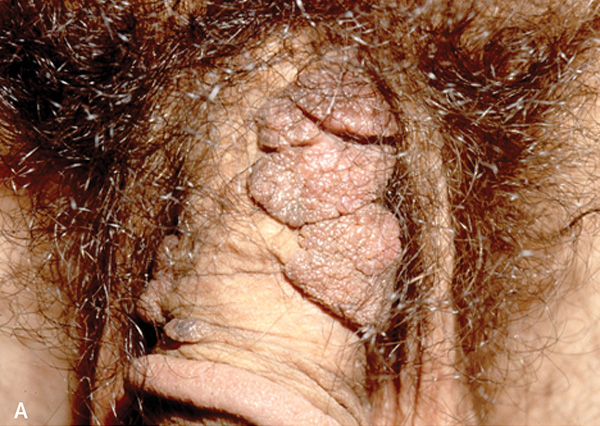
Penile cancer is rare, with a prevalence of two or three cases per 100,000 men.66 The peak incidence is in men older than 70 years.66 Squamous cell carcinoma accounts for 95% of penile cancers.63,66 Risk factors include human papillomavirus infection, lichen sclerosus, smegma, smoking, older age, poor hygiene, presence of the prepuce, and phimosis.8,31,63 Giant condyloma (Figure 8A3) may be difficult to distinguish from squamous cell carcinoma (Figure 8B3), and biopsy is indicated if the diagnosis is in doubt.
Given that, on average, patients with squamous cell carcinoma of the penis delay seeking medical care by six months or longer, presentations can vary.66,74,75 It can appear as a painless lump or ulcer that progresses to thickened skin and a wart-like growth, sometimes with a foul discharge (eFigure D and eFigure E). Rashes and skin coloration changes may occur.76 Exophytic or fungating squamous cell carcinoma typically appears as a large, irregularly shaped mass, whereas endophytic squamous cell carcinoma commonly presents as ulcerative and infiltrative lesions.66,77 Exophytic lesions occasionally lead to phimosis requiring prepuce retraction for mass visualization (eFigure F and eFigure G).
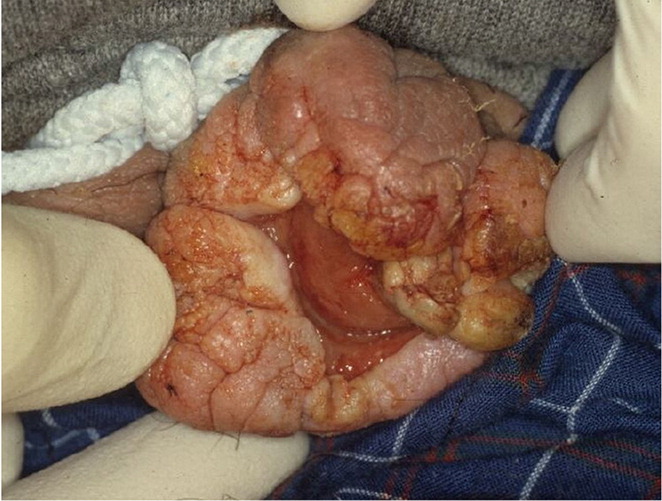
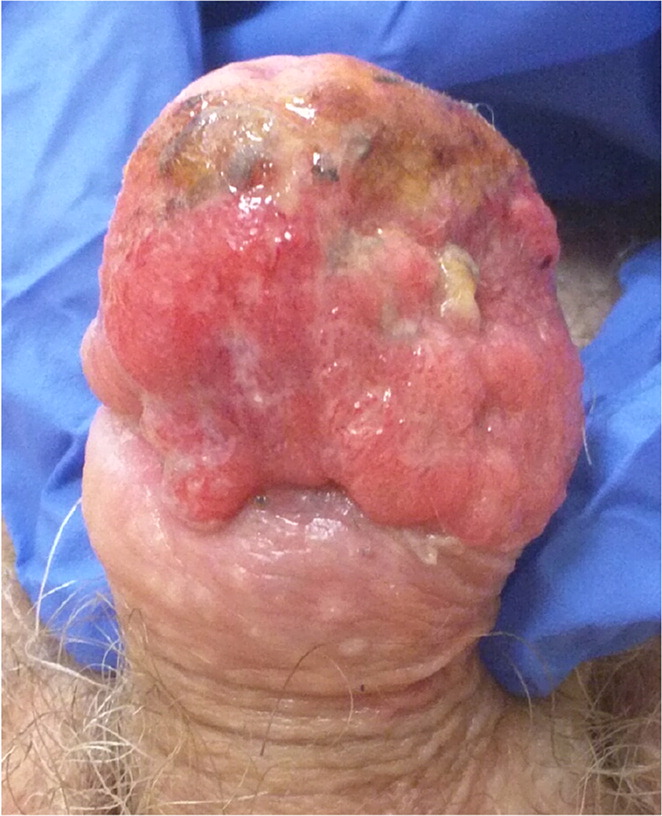
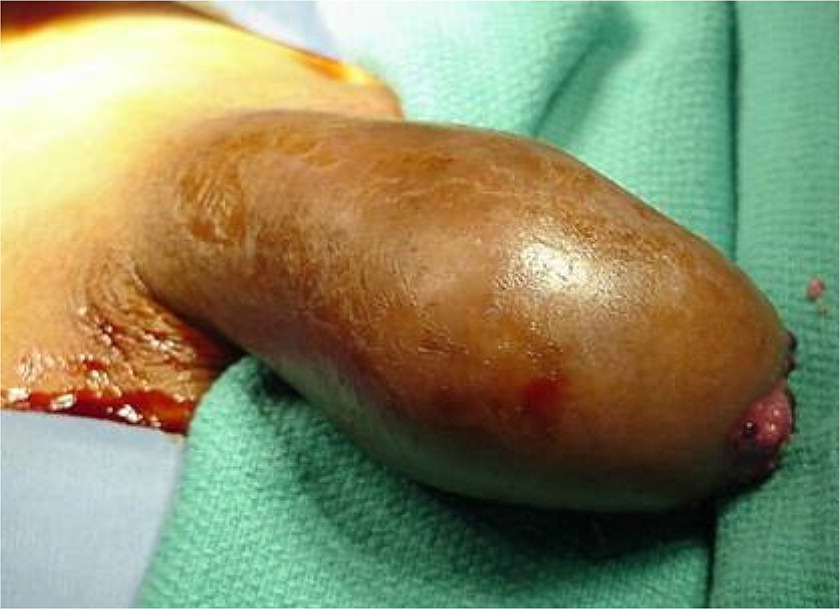
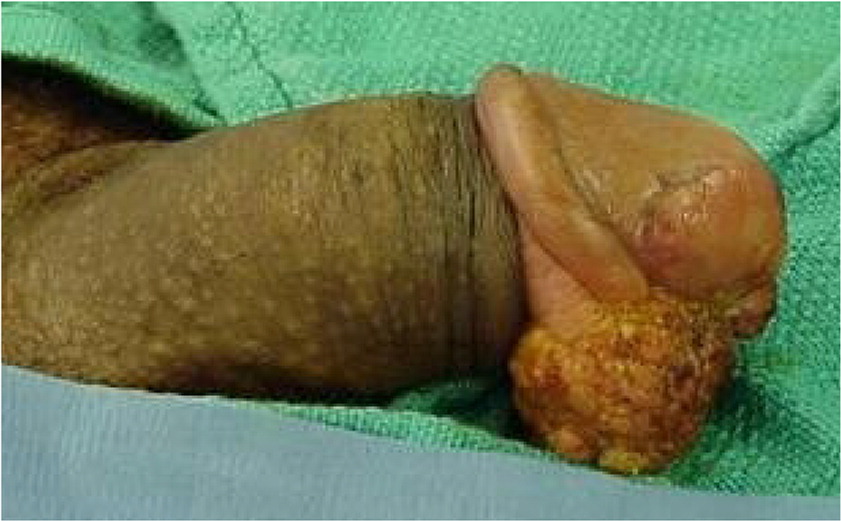
The diagnosis of squamous cell carcinoma is confirmed on biopsy.66 In general, low-grade and low-stage tumors can be treated with organ-sparing techniques, such as Mohs micrographic surgery.70 Lesions restricted to the prepuce are generally best treated with circumcision.66 Surgical excision of penile cancer with wide margins is considered the best chance for disease-free survival.78 Organ-preserving surgery is often possible.79 Partial penectomy, laser therapy, radiation, and brachytherapy have been attempted as alternatives to radical penectomy.8,63,77,80
This article updates a previous article on this topic by Teichman, et al.3
Data Sources: A PubMed search was completed in Clinical Queries using the key terms penile lesions, penile psoriasis, penile lichen sclerosus, penile lichen nitidus, penile lichen planus, penile angiokeratomas, penile carcinoma in situ, penile squamous cell carcinoma, penile squamous cell carcinoma in situ, Bowen disease, erythroplasia of Queyrat, topical corticosteroids, vitamin D analogues, tacrolimus, pimecrolimus, imiquimod. Also searched were Cochrane Database of Systematic Reviews, the National Guideline Clearinghouse, and Clinical Evidence. Last online search: November 21, 2016.
eFigures D, F, and G courtesy of Ian M. Thompson, MD, San Antonio, Tex. eFigure E courtesy of Peter C. Black, MD, Vancouver, British Columbia, Canada.