
Am Fam Physician. 2018;97(9):600-602
Author disclosure: No relevant financial affiliations.
Case Scenario
A 65-year-old East Asian woman recently shared with me the results of her 23andMe test, which revealed that she had one copy of the ɛ4 genetic variant, indicating that she has a slightly increased risk of Alzheimer disease. The test instructed her to consider talking to a health care professional if she had any symptoms of Alzheimer disease or any concerns about her test results. The test results showed she was at average risk of other genetic conditions. She is otherwise healthy, takes no medications, has no known family history of Alzheimer disease, and has no chronic conditions. She works full time as a software engineer. She reports misplacing items, such as her car keys or phone, a few times per week, and she is usually able to locate them in 15 to 30 minutes. She asked me whether she should obtain long-term care insurance given her test results.
What is the evidence for APOE genetic predictive testing for late-onset Alzheimer disease? Should these genetic test results be included in my patient's medical record? Is she protected from health or life insurance company rate discrimination? How should I advise her about long-term care insurance?
Commentary
On April 6, 2017, the U.S. Food and Drug Administration (FDA) approved marketing of the 23andMe Personal Genome Service, the first direct-to-consumer genetic test. This test uses genomic DNA collected from mail-in saliva collection kits to provide information on a patient's genetic risk of certain medical diseases or conditions. The diseases and conditions covered by 23andMe that were originally approved by the FDA include alpha1-antitrypsin deficiency, celiac disease, factor XI deficiency, Gaucher disease, glucose-6-phosphate dehydrogenase deficiency, hereditary thrombophilia, Parkinson disease, late-onset Alzheimer disease, primary dystonia, and hereditary hemochromatosis (Table 1). Currently, genetic testing for six of these disorders (alpha1-antitrypsin deficiency, celiac disease, hereditary hemochromatosis, hereditary thrombophilia, late-onset Alzheimer disease, and Parkinson disease) and age-related macular degeneration are offered in the 23andMe genetic health risk report. On March 6, 2018, the FDA approved testing for three mutations in the BRCA1 and BRCA2 genes conferring risk of hereditary breast and ovarian cancers as a separate service. Direct-to-consumer genetic testing for BRCA1/BRCA2 is not included in this discussion because, unlike the other tests mentioned, there is evidence of clinical utility for BRCA1/BRCA2 in patients with high-risk family histories1 and because the complexity of the topic warrants a separate discussion.
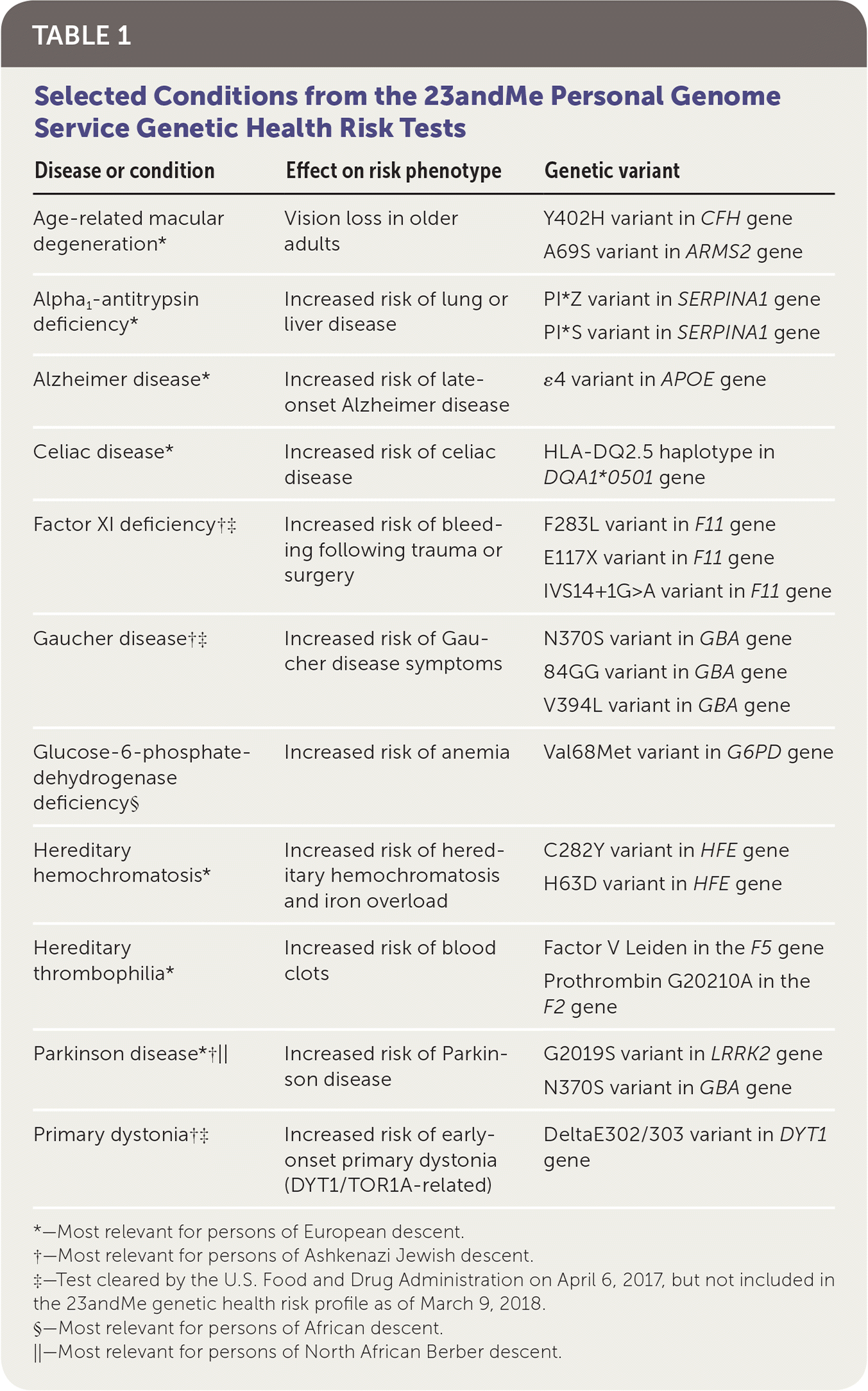
| Disease or condition | Effect on risk phenotype | Genetic variant |
|---|---|---|
| Age-related macular degeneration* | Vision loss in older adults | Y402H variant in CFH gene |
| A69S variant in ARMS2 gene | ||
| Alpha1-antitrypsin deficiency* | Increased risk of lung or liver disease | PI*Z variant in SERPINA1 gene |
| PI*S variant in SERPINA1 gene | ||
| Alzheimer disease* | Increased risk of late-onset Alzheimer disease | ɛ4 variant in APOE gene |
| Celiac disease* | Increased risk of celiac disease | HLA-DQ2.5 haplotype in DQA1*0501 gene |
| Factor XI deficiency†‡ | Increased risk of bleeding following trauma or surgery | F283L variant in F11 gene |
| E117X variant in F11 gene | ||
| IVS14+1G>A variant in F11 gene | ||
| Gaucher disease†‡ | Increased risk of Gaucher disease symptoms | N370S variant in GBA gene |
| 84GG variant in GBA gene | ||
| V394L variant in GBA gene | ||
| Glucose-6-phosphate-dehydrogenase deficiency§ | Increased risk of anemia | Val68Met variant in G6PD gene |
| Hereditary hemochromatosis* | Increased risk of hereditary hemochromatosis and iron overload | C282Y variant in HFE gene |
| H63D variant in HFE gene | ||
| Hereditary thrombophilia* | Increased risk of blood clots | Factor V Leiden in the F5 gene |
| Prothrombin G20210A in the F2 gene | ||
| Parkinson disease*†‖ | Increased risk of Parkinson disease | G2019S variant in LRRK2 gene |
| N370S variant in GBA gene | ||
| Primary dystonia†‡ | Increased risk of early-onset primary dystonia (DYT1/TOR1A-related) | DeltaE302/303 variant in DYT1 gene |
The patient in this scenario has the ɛ3/ɛ4 genotype for the APOE gene. The APOE gene encodes the apolipoprotein E protein, which is a carrier for cholesterol that is found in the central nervous system and the rest of the body. The ɛ4 variant is associated with a higher risk of developing late-onset Alzheimer disease. Individuals of European ancestry with one copy of the variant have an 18% to 20% chance of developing Alzheimer disease by 85 years of age, whereas individuals with two copies of the ɛ4 variant have a higher risk—51% to 68% by 85 years of age.2,3
The evidence supporting the association between the APOE gene and late-onset Alzheimer disease is compelling, including a genome-wide meta-analysis with a sample size of more than 70,0004; however, there are important caveats for this patient. First, the evidence is based mostly on studies of persons with European ancestry, although this association has also been reported in persons of East Asian descent.5 It is possible that the same gene influences the same phenotype (e.g., late-onset Alzheimer disease), but the clinical validity (i.e., a test's ability to identify or accurately and reliably predict the clinically defined disorder or phenotype of interest) of this genetic variant is uncertain for patients of East Asian descent.6
Second, there are no data from prospective trials for any racial/ethnic group establishing the clinical utility of genetic testing for APOE variants (i.e., evidence that testing leads to measureable improvement in clinical outcomes and that it adds value for management decision making compared with no genetic testing).6 Similar caveats apply to the other conditions covered in the 23andMe genetic health risk tests— namely, that clinical validity data are usually based on studies of European-ancestry populations and that demonstration of improved clinical outcomes has not yet been established.
The ability to use genetic health risk test results to plan for one's future may have personal utility (i.e., a test's impact on psychosocial outcomes, such as family planning, lifestyle changes, and future decision making),7 even if available evidence does not show clinical utility. In March 2017, the National Academies of Sciences, Engineering, and Medicine published a consensus study report that recommended consideration of the personal utility of a genetic test to individual patients when deciding whether to advise testing.1 Therefore, although APOE test results may not be medically actionable for Alzheimer disease treatment or cardiovascular disease prevention,5,8 the results for patients may be personally valuable if it captures actual disease risk and enables future planning and risk modification. These principles also apply to the other conditions covered in the 23andMe tests.
In 2008, the Genetic Information Nondiscrimination Act was passed, prohibiting health insurance companies from discriminating based on genetic test results.9 However, this law does not include life insurance, long-term care insurance, disability insurance, or workplace wellness programs (which can decrease health insurance premiums), and it does not protect patients with preexisting genetic conditions from rate hikes or noninsurability.10 There is evidence that persons who learn that they have at least one APOE ɛ4 allele have a higher probability of increasing their long-term care insurance.11,12 Insurance companies can ask consumers directly to disclose if they have undergone genetic testing and can request medical records before underwriting such policies. Including direct-to-consumer genetic testing results in medical records may have unintended consequences, so patients should be informed about the limits of the law's privacy protections and asked if they want to have these results included in their medical records.
For the patient in this scenario, after performing a neurologic examination and screening for cognitive decline, the physician should advise her that the results are not diagnostic for Alzheimer disease but that she is at increased risk because of her sex and APOE genotype. She should be advised that maintaining a healthy blood pressure and exercising regularly can reduce the risk of Alzheimer disease, and that it is reasonable to consider increased genetic risk as a factor in decision making regarding long-term care insurance. The physician should also advise her that at this time, the evidence has not shown improved clinical outcomes for using APOE genetic testing to prevent or treat Alzheimer disease. She should be asked if she wants to include the test results in her medical record, given that this information could be used by life and long-term care insurance companies to determine eligibility and insurance rates.
