
A more recent article on colorectal cancer screening and prevention is available.
This is an updated version of the article that appeared in print. See Editor's Note.
Am Fam Physician. 2018;97(10):658-665
Related editorials: Should Screening Techniques for Colorectal Cancer All Have an 'A' Recommendation? Yes: All Conventional Screening Techniques Should Have an 'A' Recommendation and No: When It Comes to Colorectal Cancer Screening, Test Choice Matters.
Patient information: See related handout on colon cancer, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Colorectal cancer is a common cause of morbidity and mortality in the United States. Most colorectal cancers arise from preexisting adenomatous or serrated polyps. The incidence and mortality of colorectal cancer can be reduced with screening of average-risk adults 50 to 75 years of age. Randomized controlled trials show evidence of reduced colorectal cancer–specific mortality with guaiac-based fecal occult blood tests and flexible sigmoidoscopy. There are no randomized controlled trials on the effectiveness of colonoscopy to reduce colorectal cancer–specific mortality; however, several randomized controlled trials comparing colonoscopy with other strategies are in progress. The best available evidence supporting colonoscopy is from prospective cohort studies that demonstrate decreased incidence of colorectal cancer and colorectal cancer–related mortality in individuals undergoing colonoscopy. Other screening options include fecal immunochemical testing, computed tomographic colonography, and multitargeted stool DNA testing combined with fecal immunochemical testing. There is good evidence that aspirin, nonsteroidal anti-inflammatory drugs, cyclooxygenase-2 inhibitors, and hormone therapy decrease the risk of colorectal cancer and adenomatous polyps, but potential harms limit their usefulness. There is good evidence that calcium supplementation, moderate dairy consumption, reduced red meat consumption, increased physical activity, decreased body mass index, and statin use decrease the risk of colorectal cancer and adenomatous polyps. Although increased alcohol intake and tobacco use are associated with an increased risk of colorectal cancer, there is no direct evidence that reducing alcohol consumption or smoking cessation decreases the risk.
Colorectal cancer (CRC) is the third most common cancer and cause of cancer-related deaths in the United States.1 Most CRCs are caused by adenomatous or serrated polyps as a result of sporadic mutations or DNA mismatch repair.2 CRC screening reduces mortality by removing adenomatous and serrated polyps or by early detection of CRC; however, only 62% of eligible persons were up-to-date on CRC screening, according to the 2015 National Health Interview Survey.3,4
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Colorectal cancer screening is recommended in average-risk adults 50 to 75 years of age. | A | 3 |
| Randomized controlled trials show evidence of reduced colorectal cancer–specific mortality with guaiac-based fecal occult blood testing (number needed to screen = 1,000) and flexible sigmoidoscopy screening (number needed to screen = 850). | A | 11, 25 |
| Aspirin, nonsteroidal anti-inflammatory drugs, cyclooxygenase-2 inhibitors, and hormone therapy decrease the risk of colorectal cancer and adenomatous polyps; however, increased risks of adverse effects outweigh potential benefits for most patients. | B | 28, 30–32, 52, 54 |
| Calcium supplementation, increased dairy consumption, reduced red meat consumption, increased physical activity, decreased body mass index, and statin use are associated with a lower risk of colorectal cancer and adenomatous polyps, although most of the evidence is from observational studies. | C | 37, 40, 44, 47, 48, 50–52, 55, 56 |
| There is no evidence that antioxidants, fiber, or folic acid decreases the risk of colorectal cancer or adenomatous polyps. | B | 45, 46, 49, 53 |
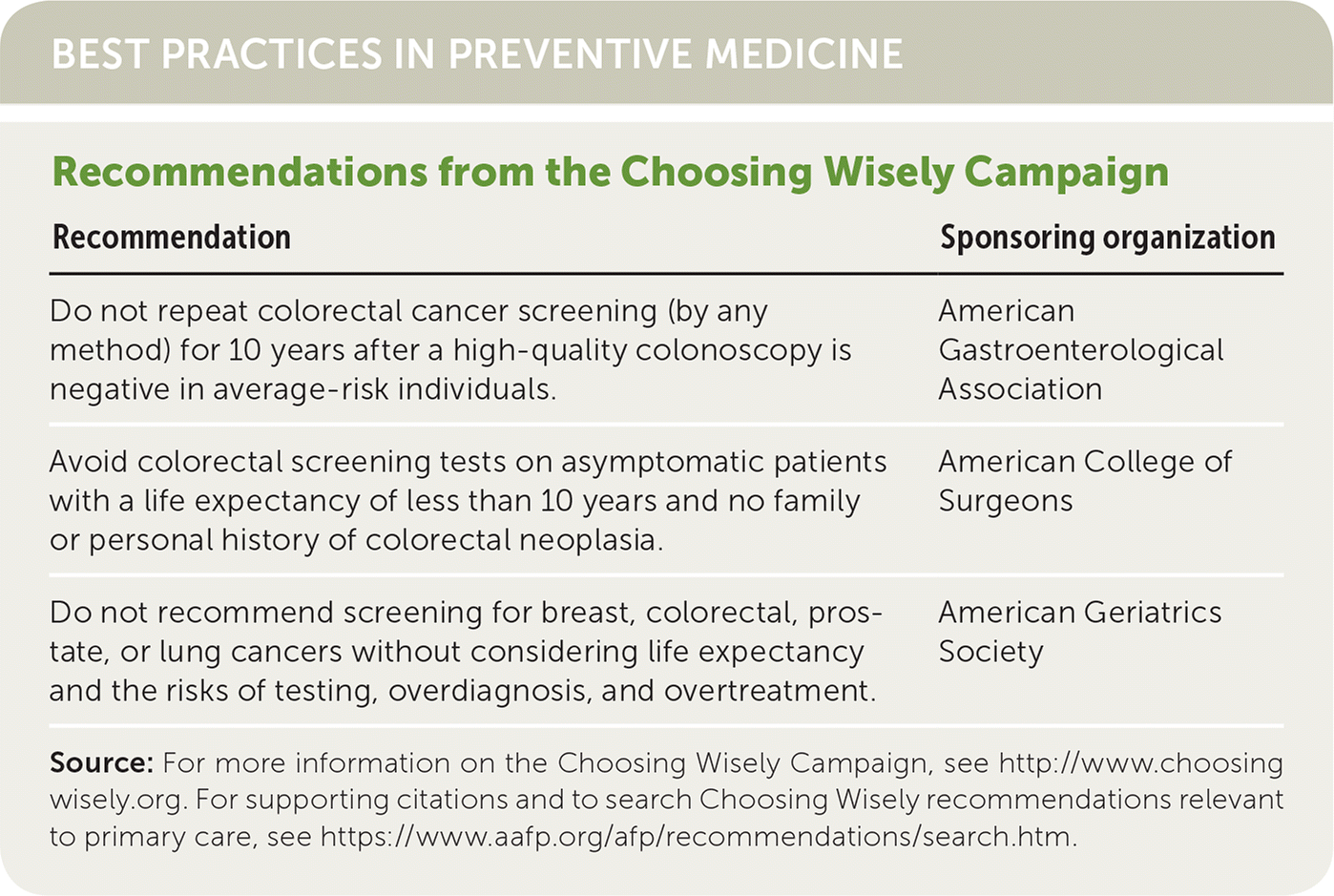
| Recommendation | Sponsoring organization |
|---|---|
| Do not repeat colorectal cancer screening (by any method) for 10 years after a high-quality colonoscopy is negative in average-risk individuals. | American Gastroenterological Association |
| Avoid colorectal screening tests on asymptomatic patients with a life expectancy of less than 10 years and no family or personal history of colorectal neoplasia. | American College of Surgeons |
| Do not recommend screening for breast, colorectal, prostate, or lung cancers without considering life expectancy and the risks of testing, overdiagnosis, and overtreatment. | American Geriatrics Society |
Screening Guidelines
The U.S. Preventive Services Task Force (USPSTF) recommends CRC screening for average-risk adults 50 to 75 years of age.3 The decision to screen adults 76 to 85 years of age should be individualized based on overall health, previous screening, willingness to undergo treatment for CRC if found, and comorbid conditions.3 Other guidelines are generally consistent (Table 1).3,5–9

| Organization | Recommendations |
| American Academy of Family Physicians5 | Screening for CRC with FIT, flexible sigmoidoscopy, or colonoscopy should start at 50 years of age and continue until 75 years of age. The decision to screen for CRC in adults 76 to 85 years of age should be individualized, taking into account the patient's overall health and screening history. Screening for CRC is not recommended in adults older than 85 years. |
| American Cancer Society6 | Starting at 45 years of age and continuing through the age of 75 years, adults at average risk of CRC with a life expectancy of more than 10 years should undergo regular screening with either a high-sensitivity stool-based test or a structural (visual) examination, depending on patient preference and test availability. Clinicians should individualize screening decisions for individuals aged 76 through 85 years, and discourage individuals older than 85 years from continuing CRC screening.
|
| American College of Gastroenterology7 | The preferred test is colonoscopy every 10 years, beginning at 50 years of age. Screening should begin at 45 years of age in blacks. FIT should be offered to patients who decline colonoscopy. |
| Canadian Task Force on Preventive Health Care8 | Screening for CRC should start at 50 years of age and continue until 74 years of age using stool-based tests or direct visualization tests. Stool-based test: gFOBT or FIT every two years Direct visualization test: Flexible sigmoidoscopy every 10 years Colonoscopy is not recommended as a screening test for CRC. |
| U.S. Multi-Society Task Force on Colorectal Cancer9 | Screening for CRC should begin at 50 years of age in average-risk persons; however, limited evidence supports screening beginning at 45 years of age in blacks. Discontinue screening at 75 years of age or in individuals who have a life expectancy less than 10 years. First-tier recommendation: Colonoscopy every 10 years or annual FIT Second-tier recommendation: Computed tomographic colonography every five years, FIT-DNA test every three years, or flexible sigmoidoscopy every five to 10 years Third-tier recommendation: Capsule colonoscopy every five years |
| U.S. Preventive Services Task Force3 | Screening for CRC should start at 50 years of age and continue until 75 years of age.
|
The U.S. Food and Drug Administration recently approved a new serology test to detect circulating methylated SEPT9 DNA (Epi proColon), which is found in some CRCs.3 A prospective study of 7,941 individuals found poor sensitivity (48%) with this test,10 and it is not recommended by any U.S. cancer screening guidelines.
Stool-Based Screening
Stool-based screening is quick, noninvasive, and can be done in the home.3 Stool-based screening methods include guaiac-based fecal occult blood test (gFOBT), fecal immunochemical test (FIT), and multitargeted stool DNA test (FIT-DNA).3 Of these, FIT-DNA is significantly more expensive than the other two.
gFOBT is the most common stool-based test for CRC screening worldwide. A 2013 Cochrane review of four randomized controlled trials (RCTs) involving 329,642 individuals concluded that, compared with no screening, gFOBT screening reduced CRC-specific mortality after 11 to 30 years of follow-up (number needed to screen = 1,000).11 gFOBT has fair sensitivity (62% to 79%) but good specificity (87% to 96%) for detecting CRC (positive likelihood ratio [LR+] = 4.7 to 19.8; negative likelihood ratio [LR–] = 0.2 to 0.4).3 Screening with gFOBT should be performed yearly.3 Individuals must submit three stool samples collected at home, and avoid heme-containing foods before the test. Office-based gFOBT is not recommended for CRC screening.
A 2014 meta-analysis of 19 cohort studies found that FIT is more sensitive and specific than gFOBT for detecting CRC.12 FIT also has the advantages of not requiring any dietary restrictions and needing only a single stool sample. One RCT found that screening with immunochemical fecal occult blood testing had no significant impact on CRC mortality (relative risk = 0.88; 95% confidence interval [CI], 0.72 to 1.07).13 FIT has good sensitivity (73% to 88%) and excellent specificity (91% to 95%) for detecting CRC (LR+ = 8.1 to 17.6; LR– = 0.1 to 0.3).3 Screening with FIT also should be performed yearly.3
A FIT-DNA assay (ColoGuard) combines FIT with testing for altered DNA biomarkers.14 A prospective study of 9,989 persons 50 to 84 years of age who underwent screening colonoscopy found that FIT-DNA was more sensitive (92% vs. 74%) but less specific (90% vs. 96%) than FIT alone for detecting CRC.15 However, both FIT and FITDNA have poor sensitivity (24% and 42%, respectively) for detecting adenomatous polyps and serrated polyps measuring 1 cm or greater.3 Screening with FIT-DNA should be performed every one to three years, but it is reimbursed by Medicare only every three years.3
Direct Visualization Screening
Direct visualization tests, which include colonoscopy, computed tomographic colonography (CTC ; also called virtual colonoscopy), and flexible sigmoidoscopy, allow for the identification of adenomatous polyps and serrated polyps before their natural progression to CRC. Polyps found during colonoscopy can be removed during the procedure, whereas polyps found using other methods require follow-up colonoscopy. Harms may occur during bowel preparation (e.g., dehydration, electrolyte imbalances), sedation or anesthesia for colonoscopy (e.g., cardiovascular events), or the procedure itself (e.g., colonic perforations, bleeding).
Colonoscopy, the most widely used test for CRC screening in the United States, is typically performed in a hospital-based endoscopy suite or an ambulatory surgical center. It involves complete bowel preparation (e.g., polyethylene glycol [Golytely]), and is typically performed with sedation. There are no RCTs on colonoscopy to reduce CRC-specific mortality; however, several RCTs comparing colonoscopy with FIT or no screening are in progress.16,17 A 2013 prospective cohort study of 88,902 participants in the Nurses' Health Study reported a 27% decreased incidence of CRC and a 68% decrease in mortality from CRC in individuals who had undergone a colonoscopy.18 Screening with colonoscopy is recommended every 10 years in average-risk persons with normal findings and good bowel preparation.3
Serious risks of colonoscopy include cardiopulmonary complications (0.9%), bowel perforation (less than 0.1%), hemorrhage (0.1% to 0.6%), infection (less than 0.1%), and postpolypectomy syndrome (2.9%).19 Postpolypectomy syndrome is characterized by abdominal pain, leukocytosis, and peritoneal inflammation in the absence of perforation after polypectomy with electrocoagulation; it may occur up to two weeks after polypectomy. A systematic review of six studies with 465 individuals found that the miss rate after colonoscopy was 2.1% for adenomatous polyps greater than 10 mm and 26% for adenomatous polyps 1 to 5 mm.20 A population-based cohort study of 12,487 individuals reported the miss rate was 2% to 6% for CRCs.21
CTC is an alternative screening test, but supporting evidence is limited to studies of its test characteristics. Radiation exposure from a single CTC is equivalent to 70 chest radiographs.22,23 When used for screening, CTC requires bowel preparation but not administration of intravenous contrast media. A 2011 meta-analysis of five RCTs or cohort studies comparing CTC with colonoscopy in average-risk adults 50 years and older found that CTC has high sensitivity for adenomatous polyps greater than 10 mm.24 Extracolonic findings have been reported in up to 70% of examinations, which is a potential harm because only 3% of these findings require treatment.3 CTC has good sensitivity (67% to 94%) and very good to excellent specificity (86% to 98%) for detecting adenomatous polyps and serrated polyps measuring 1 cm or greater (LR+ = 4.8 to 47; LR– = 0.06 to 0.4).3 Screening with CTC is recommended every five years.3
Flexible sigmoidoscopy may be performed in the office without sedation, but it requires bowel preparation. A 2013 Cochrane review of five RCTs with 414,754 individuals evaluated the effectiveness of flexible sigmoidoscopy in average-risk adults compared with no screening or with usual care.11 This review found that, compared with no screening, flexible sigmoidoscopy lowers CRC mortality by 28% (95% CI, 21% to 35%).11 A meta-analysis of five RCTs concluded that flexible sigmoidoscopy reduces the incidence of CRC (number needed to screen = 361 to prevent one case of CRC) and reduces CRC mortality (number needed to screen = 850).25 One RCT including 98,792 individuals comparing flexible sigmoidoscopy plus gFOBT with flexible sigmoidoscopy alone concluded that there was no benefit to adding gFOBT in terms of CRC incidence or mortality.26 A pooled analysis of three large RCTs including 287,928 individuals found that flexible sigmoidoscopy reduced the incidence of CRC and CRC mortality in men and in women younger than 60 years; however, this study concluded that alternative screening methods should be considered in women older than 60 years.27 The USPSTF recommends screening with flexible sigmoidoscopy every five years.3
Choosing a Test: Benefits, Harms, Burdens, and Costs
Family physicians can help their patients choose a test for CRC screening by reviewing test characteristics, benefits, harms, burdens, and costs. In addition to the advantages and disadvantages of each test, physicians must consider patient preference, comorbidities, test availability, likelihood that the test will be completed, and availability of resources for follow-up of abnormal test results. Table 2 and Table 33 provide an overview of the tests recommended by the USPSTF. All approaches are thought to significantly decrease deaths caused by CRC (between 20 and 24 per 1,000 persons screened, depending on the test), although complication rates and lifetime colonoscopy burden vary considerably.
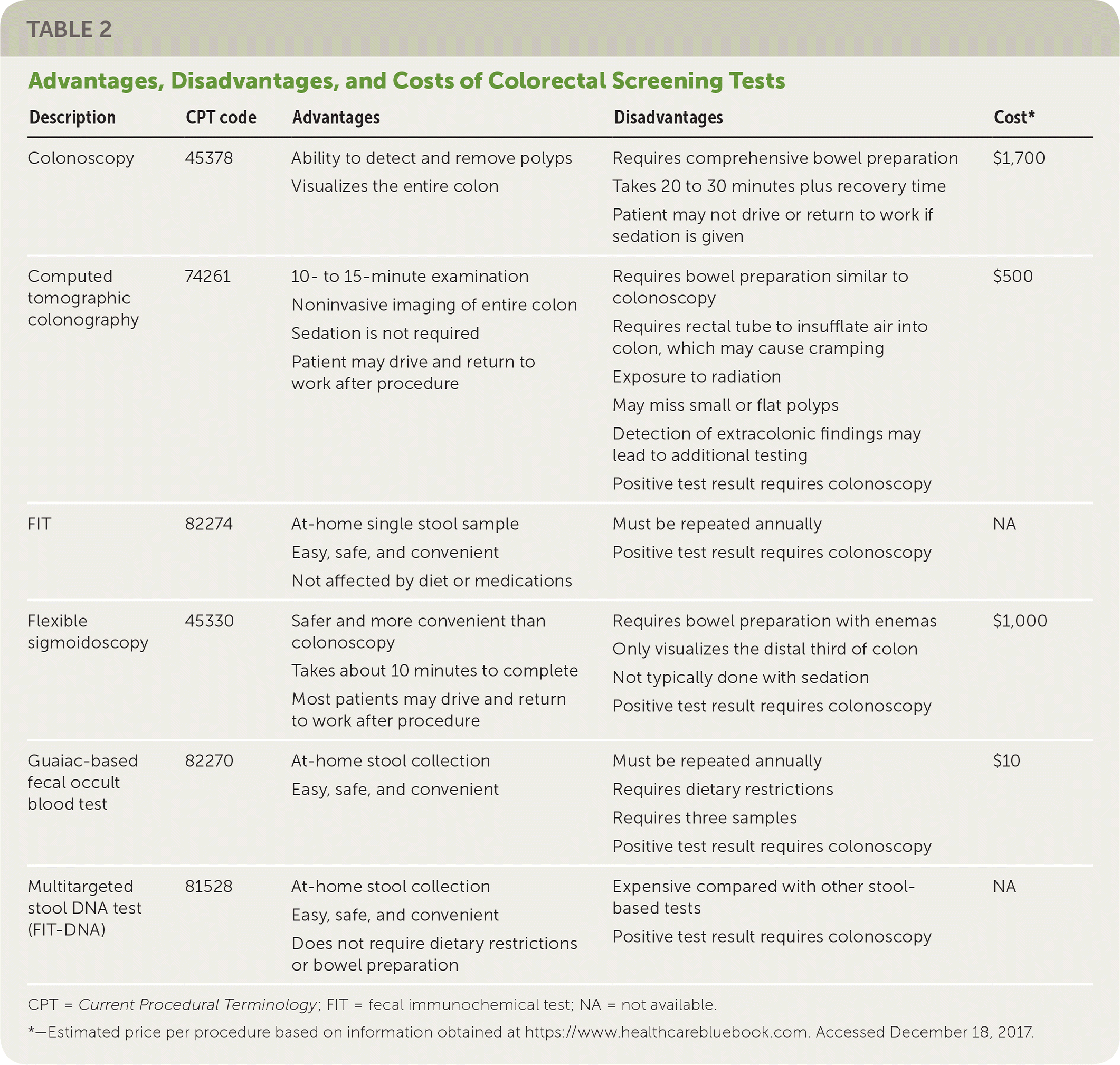
| Description | CPT code | Advantages | Disadvantages | Cost* |
|---|---|---|---|---|
| Colonoscopy | 45378 | Ability to detect and remove polyps Visualizes the entire colon | Requires comprehensive bowel preparation Takes 20 to 30 minutes plus recovery time Patient may not drive or return to work if sedation is given | $1,700 |
| Computed tomographic colonography | 74261 | 10- to 15-minute examination Noninvasive imaging of entire colon Sedation is not required Patient may drive and return to work after procedure | Requires bowel preparation similar to colonoscopy Requires rectal tube to insufflate air into colon, which may cause cramping Exposure to radiation May miss small or flat polyps Detection of extracolonic findings may lead to additional testing Positive test result requires colonoscopy | $500 |
| FIT | 82274 | At-home single stool sample Easy, safe, and convenient Not affected by diet or medications | Must be repeated annually Positive test result requires colonoscopy | NA |
| Flexible sigmoidoscopy | 45330 | Safer and more convenient than colonoscopy Takes about 10 minutes to complete Most patients may drive and return to work after procedure | Requires bowel preparation with enemas Only visualizes the distal third of colon Not typically done with sedation Positive test result requires colonoscopy | $1,000 |
| Guaiac-based fecal occult blood test | 82270 | At-home stool collection Easy, safe, and convenient | Must be repeated annually Requires dietary restrictions Requires three samples Positive test result requires colonoscopy | $10 |
| Multitargeted stool DNA test (FIT-DNA) | 81528 | At-home stool collection Easy, safe, and convenient Does not require dietary restrictions or bowel preparation | Expensive compared with other stool-based tests Positive test result requires colonoscopy | NA |
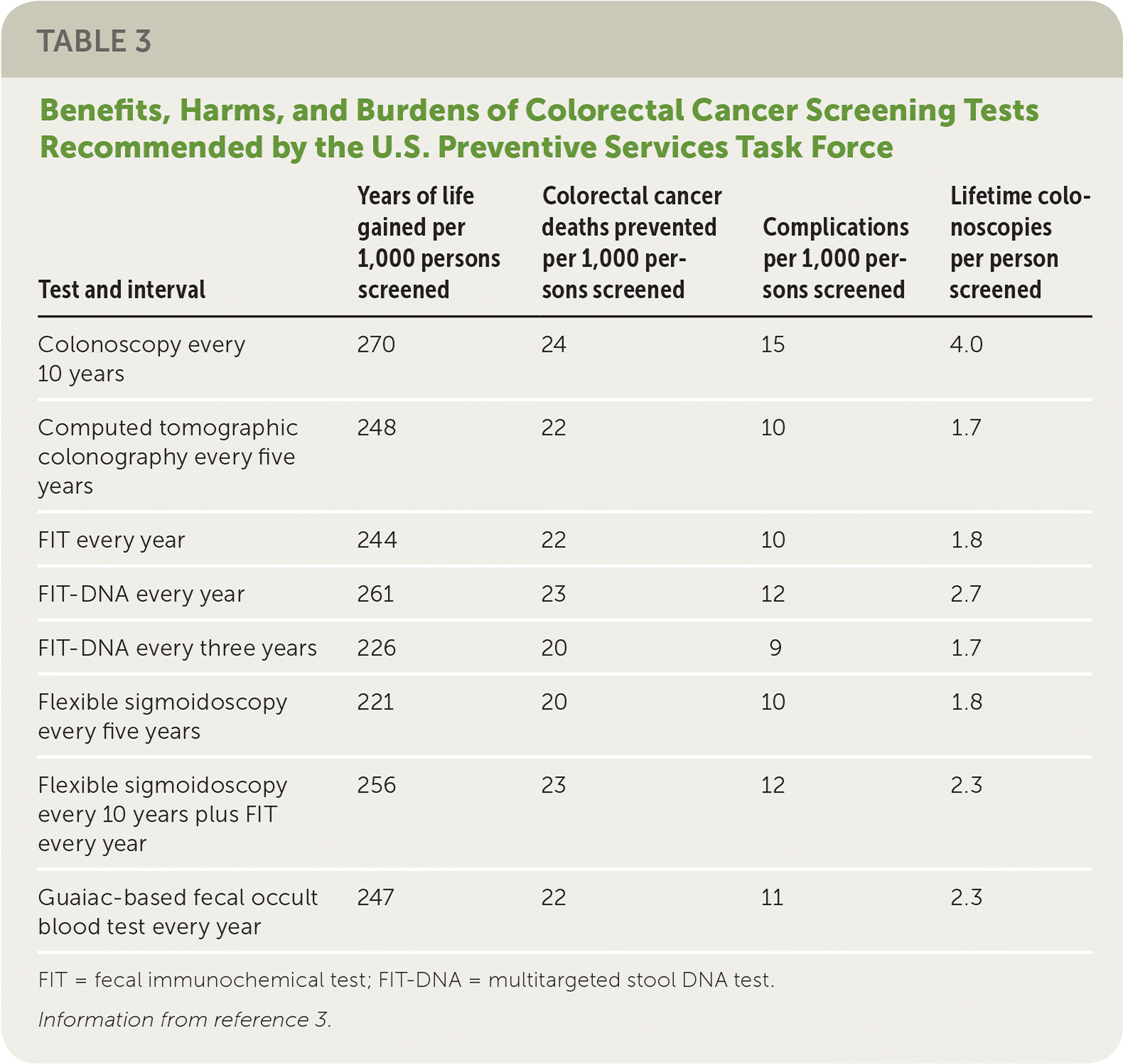
| Test and interval | Years of life gained per 1,000 persons screened | Colorectal cancer deaths prevented per 1,000 persons screened | Complications per 1,000 persons screened | Lifetime colonoscopies per person screened |
|---|---|---|---|---|
| Colonoscopy every 10 years | 270 | 24 | 15 | 4.0 |
| Computed tomographic colonography every five years | 248 | 22 | 10 | 1.7 |
| FIT every year | 244 | 22 | 10 | 1.8 |
| FIT-DNA every year | 261 | 23 | 12 | 2.7 |
| FIT-DNA every three years | 226 | 20 | 9 | 1.7 |
| Flexible sigmoidoscopy every five years | 221 | 20 | 10 | 1.8 |
| Flexible sigmoidoscopy every 10 years plus FIT every year | 256 | 23 | 12 | 2.3 |
| Guaiac-based fecal occult blood test every year | 247 | 22 | 11 | 2.3 |
Prevention
eTable A summarizes colon cancer prevention strategies, including medications, lifestyle factors, and diet.
| Factor | Effect | Comment | Evidence |
|---|---|---|---|
| Diet | |||
| Cholesterol and fat intake | Twofold increased risk of CRC with increased cholesterol intake, and 25% increased risk of serrated polyps with increased fat intake | There is no evidence that reduction in cholesterol or fat intake lowers risk of CRC or serrated polyps | One meta-analysis, one RCT, and one prospective studyA1–A3 |
| Coffee consumption | Conflicting evidence | More research is needed from high-quality trials | One case-control study, one RCT, and one meta-analysis of prospective cohort studesA4–A6 |
| Dairy intake | 15% reduced risk of CRC with more than 8 oz of cow's milk per day | Moderate intake of cow's milk reduced risk of CRC | Meta-analysis of 10 cohort studiesA7 |
| Fiber | Increased fiber intake does not reduce the risk of CRC or recurrent adenomatous polyps | Fiber from different sources was used | Two Cochrane reviews and one meta-analysis of 13 prospective studiesA8–A10 |
| Red meat intake | 22% increased risk of CRC with increasing red meat and processed meat intake | For every 3.5 oz of red meat intake per day, there was a 14% increased risk of CRC | Meta-analysis of 13 prospective studies and meta-analysis of 34 case-control studies and 14 cohort studiesA11,A12 |
| Lifestyle | |||
| Alcohol intake | 8% increased risk of CRC and 24% increased risk of serrated polyps | Evaluated effect of > 15 g per day of beer, wine, or spirits | Large cohort study (CRC) and meta-analysis of 10 observational studies (serrated polyps)A13,A14 |
| Cigarette smoking | 114% increased risk of high-risk adenomatous polyps and CRC in current smokers | Strong association between smoking and high-risk adenomatous polyps and CRC; no direct evidence that smoking cessation decreases risk | Meta-analysis of 42 observational studiesA15 |
| Obesity | Bariatric surgery associated with 27% reduced risk of CRC | Increasing body mass index associated with increased risk of colon cancer but not rectal cancer | Meta-analysis of fourobservational studies and a systematic review of 15 cohort studiesA16–A18 |
| Physical activity | 26% decreased risk of colon cancer for occupational physical activity, and 20% decreased risk of colon cancer with recreational physical activity 12% decreased risk of rectal cancer for occupational physical activity, and 13% decreased risk of rectal cancer with recreational physical activity | Demonstrated benefit of increasing occupational and recreational physical activity for reducing the risk of colon and rectal cancers | Meta-analysis of 17 cohort studies and 21 case-control studiesA19 |
| Medications | |||
| Antioxidants | No benefit for beta carotene; vitamins A, C, or E; or selenium | Not recommended to decrease the risk of CRC or adenomatous polyps | Meta-analysis of 20 RCTsA20 |
| Aspirin | 40% reduced risk of CRC | Not recommended in the average-risk population because of the risk of GI bleeding and hemorrhagic stroke | Review of three RCTsA21,A22 |
| Calcium | 26% reduced risk of adenomatous polyps and 22% reduced risk of CRC; no effect for serrated polyps | Calcium use for three to four years is recommended to decrease risk of CRC or adenomatous polyps but not serrated polyps | Cochrane review and meta-analysisA1,A23,A24 |
| Cyclooxygenase-2 inhibitors | 33% reduction of adenomatous polyps with use of celecoxib, and 35% reduction with use of rofecoxib (withdrawn from the market over safety concerns) | Not recommended secondary to increased risk of GI and cardiovascular events | Two RCTsA25–A27 |
| Folic acid | Increased folic acid intake does not decrease risk of adenomatous polyps | Study examined effect of folic acid (1 mg per day) compared with placebo | RCTA28 |
| Hormone therapy | 63% reduced risk of CRC but no decreased risk of serrated polyps | Harms outweigh potential benefits, and routine use of hormone therapy is not recommended at this time | Meta-analysis of four studies and one case-control studyA1,A29 |
| Nonsteroidal anti-inflammatory drugs | 63% decreased risk of CRC | Not recommended secondary to increased risk of GI and cardiovascular events | Meta-analysis of 15 RCTsA30 |
| Statins | Statin use associated with 17% decreased risk of advanced adenomatous polyps and 50% decreased risk of CRC | Effect observed in individuals who had used a statin for at least five years | Meta-analysis of six studies and one case-control studyA31,A32 |
| Vitamin D | Guideline from U.S. Preventive Services Task Force found no benefit in vitamin D supplementation to decrease risk of CRC; a meta-analysis found 50% decreased risk of CRC or adenomatous polyps | Conflicting evidence that vitamin D supplementation decreases risk of CRC or adenomatous polyps | Guideline based on three RCTs and 28 observational studies, and meta-analysis of 18 observational studiesA33,A34 |
ASPIRIN
Although aspirin use decreases CRC incidence by 40%, a 2016 USPSTF guideline including three RCTs recommended against aspirin use in the average-risk population because of the risk of gastrointestinal bleeding and hemorrhagic stroke.28 Individuals 50 to 59 years of age with a 10-year cardiovascular event risk of at least 10% who are willing to take aspirin for at least 10 years (i.e., the time it takes to accrue the cancer prevention benefit) may benefit from aspirin use for CRC risk reduction. A 2017 systematic review with meta-analysis found that the effect of aspirin was similar to FOBT and flexible sigmoidoscopy for reducing CRC incidence and mortality, and aspirin was more effective for cancers in the proximal colon.29
NONSTEROIDAL ANTI-INFLAMMATORY DRUGS AND CYCLOOXYGENASE-2 INHIBITORS
LIFESTYLE FACTORS
Although studies have found an association between CRC and increased alcohol intake,34,35 there is no direct evidence that decreasing alcohol consumption decreases the risk of CRC, adenomatous polyps, or serrated polyps. Cigarette smoking is associated with an increased risk of CRC and adenomatous polyps; a 2008 meta-analysis found that current smokers were more than twice as likely to develop high-risk adenomatous polyps or CRC.36 However, there is a lack of evidence that smoking cessation decreases the risk of CRC, adenomatous polyps, or serrated polyps.
Reduction of body mass index may decrease the risk of CRC. A 2007 meta-analysis found an association between increasing body mass index and CRC,37 and a 2014 meta-analysis found that bariatric surgery was associated with a 27% decreased risk of CRC compared with individuals with obesity who did not undergo the surgery.38 Higher body mass index is also a risk factor for CRC mortality (hazard ratio = 1.03; 95% CI, 1.00 to 1.05).39
Increased physical activity is also associated with reduced risk of CRC. A 2017 meta-analysis showed that increased occupational and recreational activity were associated with a reduced risk of CRC.40
DIET
Although there is an association between increased fat intake and the risk of serrated polyps, there is no evidence that low-fat diets decrease risk. An RCT including 48,835 postmenopausal women 50 to 75 years of age found that women assigned to a low-fat diet did not have a lower risk of CRC than those assigned to usual diets after an eight-year follow-up.41
There is conflicting evidence on coffee consumption and the risk of CRC. The largest, most recent, and best-designed study is the Women's Health Initiative with 83,778 individuals. It found that moderate and high coffee intake (four or more cups per day) were associated with a small increase in the risk of CRC (hazard ratio = 1.14 to 1.15).42 However, another large meta-analysis of prospective cohort studies including 2,046,575 individuals found that the risk of colon cancer was decreased by 7% for every four cups of coffee consumed per day (relative risk = 0.93; 95% CI, 0.88 to 0.99; P = .199).43 Moderate intake of dairy (8 oz or more per day) is associated with a small decrease in the risk of CRC based on a 2004 meta-analysis.44 Because of processing, it is difficult to separate data on dairy products from data on vitamin D and calcium.
Two Cochrane reviews found that increasing fiber intake did not lower the risk of CRC or recurrence of adenomatous polyps.45,46 However, a reduction in red meat consumption is associated with a decreased risk of CRC. Previous meta-analyses found a modest increase in the risk of CRC associated with increased red meat consumption.47,48
OTHER MEDICATIONS AND VITAMINS
Antioxidants should not be recommended to decrease the risk of CRC or adenomatous polyps. A 2013 meta-analysis of 20 RCTs including 268,590 individuals found that, compared with no treatment or placebo, there was no benefit of antioxidant use for reducing the risk of CRC or adenomatous polyps.49
Calcium supplementation decreases the risk of adenomatous polyps and is associated with a decreased risk of CRC in observational studies. A 2005 Cochrane review identified two randomized trials with 1,346 participants, and found a significant reduction in recurrent adenomatous polyps with calcium supplementation (odds ratio = 0.74; 95% CI, 0.58 to 0.95).50 A 2016 analysis of two cohort studies demonstrated that total calcium intake of at least 1,400 mg per day compared with less than 600 mg per day was associated with a 22% reduced risk of CRC.51 A 2017 meta-analysis that examined the effect of calcium on serrated polyps found no statistically significant effect.52
There is no evidence to support folic acid supplementation to decrease the risk of adenomatous polyps.53 Although oral hormone therapy has been associated with a decreased risk of CRC in a case-control study, the harms outweigh potential benefits, and routine use is not recommended.54 A 2017 meta-analysis did not find a statistically significant association between hormone therapy and the risk of serrated polyps.52
There is some evidence from observational studies that statin use is associated with a lower risk of advanced adenomatous polyps and CRC. However, data from RCTs are lacking.55,56 Finally, there is conflicting evidence whether vitamin D supplementation decreases the risk of CRC or adenomatous polyps. A 2011 USPSTF report found no change in CRC risk in patients taking a vitamin D supplement.57
This article updates previous articles on this topic by Short, et al.58 ; Wilkins and Reynolds59 ; and Pignone and Levin.60
Data Sources: A clinical librarian completed a general PubMed search using the following MeSH terms: mass screening, colonoscopy, colorectal neoplasms, adenomatous polyp, colorectal cancer, primary prevention, and secondary prevention. These terms were also used as key words in a number of combinations. The search included meta-analyses, randomized controlled trials, and practice guidelines within the previous 20 years, and was expanded to reviews and clinical trials where needed. Reviews were hand-searched for further articles. Also searched were the Cochrane database and Essential Evidence Plus. Search dates: October 2016 and December 2017.
Editor's Note: Table 1 of this article has been updated to reflect the American Cancer Society (ACS) guideline published on May 30, 2018. Based on the results of modeling analyses, the ACS suggests starting screening for colorectal cancer in average-risk adults at age 45 years. All other organizations, including the U.S. Preventive Services Task Force and the American Academy of Family Physicians, recommend starting screening at age 50 years.
