
Am Fam Physician. 2019;100(1):16-17
Author disclosure: No relevant financial affiliations.
Clinical Question
Which noninvasive diagnostic test—urea breath test, serology, or stool antigen test—provides the most accurate diagnosis of Helicobacter pylori infection in symptomatic and asymptomatic patients?
Evidence-Based Answer
When compared with serology or stool antigen tests, the urea breath test has the highest diagnostic accuracy to identify H. pylori infection in patients without a history of gastrectomy or recent use of antibiotics or proton pump inhibitors. Use of any of these three methods in a hypothetical cohort with an H. pylori prevalence of 53.7% and fixed specificity of 90% resulted in 46 false-positive results out of 1,000 patients tested, and the urea breath test had the lowest false-negative rate.1 (Strength of Recommendation: B, based on inconsistent or limited-quality patient-oriented evidence.)
Practice Pointers
H. pylori infection affects 50% of the world's population.2 In the United States, the seroprevalence of H. pylori is higher in blacks (53%) and Mexicans (62%) than in whites (26%), and it is more common in groups with lower socioeconomic status.3 H. pylori infection can cause dyspepsia, peptic ulcer disease, and gastric cancers and is associated with iron deficiency anemia, idiopathic thrombocytopenia, and colorectal adenomas. Diagnostic tests used in the primary care office include serology and stool antigen tests, whereas the urea breath test and gastric biopsies require subspecialist referral. Once H. pylori has been detected, the treatment with standard therapy results in a 70% to 85% eradication rate.4
This review included 101 diagnostic studies involving a total of 11,003 patients, 5,839 (53%) of whom had confirmed H. pylori infection.1 The authors evaluated the diagnostic accuracy of the urea breath test-13C, urea breath test-14C, serology studies, and stool antigen tests. The reference standard was endoscopic biopsy with pathognomonic staining in the same patient. Studies included adults and children (1,508 children in 14 studies) with and without gastrointestinal symptoms, and most studies excluded patients with gastrectomy and recent (not defined) use of antibiotics or proton pump inhibitors. Eight of the studies (with 843 patients) were completed in North America; none of these studies included children. All but one study had a moderate to high risk of bias because of a lack of consecutive or random patient selection, lack of blinding, and variable test diagnostic thresholds.
The urea breath test had the highest diagnostic accuracy and lowest false-negative rate for the detection of H. pylori. To enable comparison of the tests in a hypothetical sample of 1,000 patients, the authors calculated false-negative rates at a prevalence of 53.7% and used a median specificity of 90%. At these fixed values, all four tests had a false-positive rate of 46 per 1,000, whereas the urea breath test had the lowest false-negative rate and the stool antigen test had the highest. The positive predictive values were similar.
The American College of Gastroenterology (ACG) recommends testing in patients with active peptic ulcer disease, dyspepsia symptoms, and gastric mucosa–associated lymphoid tissue lymphoma, whereas the Maastricht guidelines also include patients starting long-term nonsteroidal anti-inflammatory drugs, those with idiopathic thrombocytopenic purpura, and those who desire testing.5,6 The ACG states that the urea breath test has the highest sensitivity and specificity overall but, in a low-prevalence population (around 20%), stool antigen testing also performs well. The ACG does not recommend serology testing in a low-prevalence population because of a high false-positive rate. Urea breath testing is easily attainable and can make a rapid diagnosis, although its availability is often limited to subspecialty clinics and can cost $150 to $400. Family physicians should test all patients with dyspepsia for H. pylori, and although the urea breath test offers the lowest false-negative rates, the false-negative rates of the more readily available serology and stool antigen tests are only slightly higher.
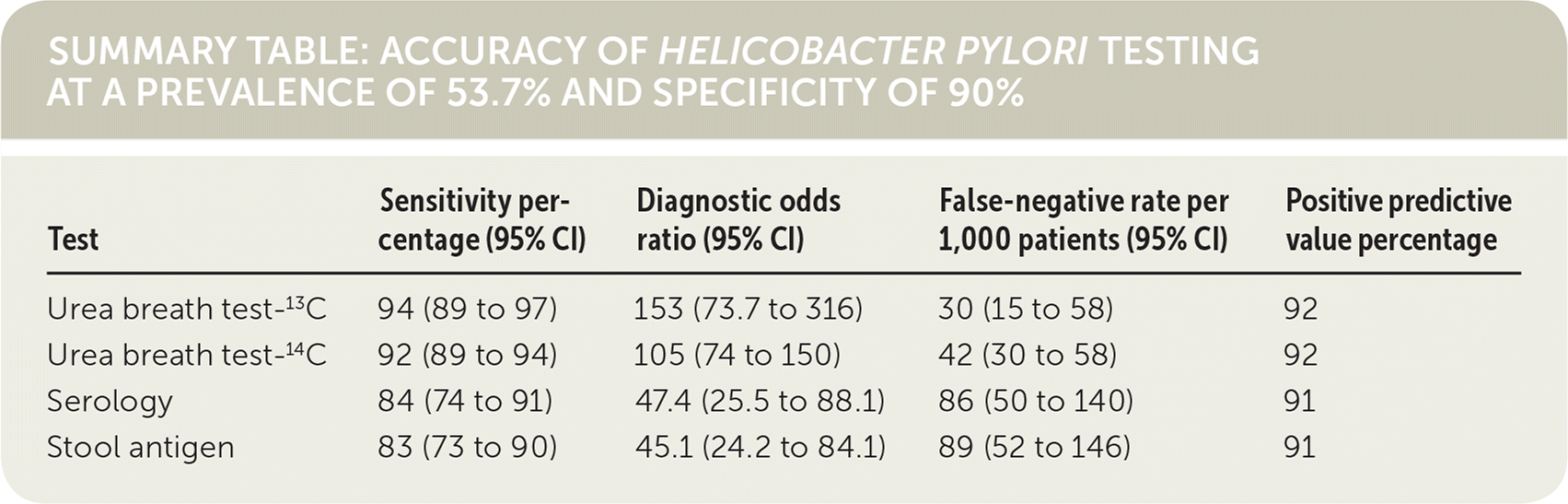
| Test | Sensitivity percentage (95% CI) | Diagnostic odds ratio (95% CI) | False-negative rate per 1,000 patients (95% CI) | Positive predictive value percentage |
|---|---|---|---|---|
| Urea breath test-13C | 94 (89 to 97) | 153 (73.7 to 316) | 30 (15 to 58) | 92 |
| Urea breath test-14C | 92 (89 to 94) | 105 (74 to 150) | 42 (30 to 58) | 92 |
| Serology | 84 (74 to 91) | 47.4 (25.5 to 88.1) | 86 (50 to 140) | 91 |
| Stool antigen | 83 (73 to 90) | 45.1 (24.2 to 84.1) | 89 (52 to 146) | 91 |
The practice recommendations in this activity are available at http://www.cochrane.org/CD012080.
Editor's Note: The positive predictive values reported in this Cochrane for Clinicians were calculated by the authors based on raw data provided in the original Cochrane review.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Navy, the Defense Health Agency, the Department of Defense, or the U.S. government.
