
Am Fam Physician. 2019;100(3):143-146
Author disclosure: No relevant financial affiliations.
Clinical Question
Do influenza vaccines reduce the risk of influenza in healthy children, healthy adults, and older adults?
Evidence-Based Answer
Influenza vaccination reduces rates of laboratory-confirmed influenza and symptomatic influenza-like illness in healthy children, healthy adults, and older adults. There is no consistent evidence that influenza vaccination reduces school absenteeism in children, parental absenteeism from work, or adult hospitalizations, nor is there conclusive evidence that influenza vaccination decreases mortality.1–3 (Strength of Recommendation: A, based on consistent, good-quality patient-oriented evidence.)
Practice Pointers
Most people infected with influenza or viruses causing influenza-like illness (i.e., conditions that produce similar symptoms, including fever, headache, muscle aches, cough, and rhinorrhea) recover without long-term sequelae. However, serious illness and death can occur, especially among young children, pregnant women, those with chronic medical conditions, and older adults.4 Less than 25% of patients with influenza-like illness test positive for confirmed infection with viral influenza,5 so much of the morbidity attributed to influenza may be caused by other viruses. The Cochrane Library simultaneously published three reviews that stratified patients into cohorts of children, adults, and older adults.1–3 The authors of these reviews sought to determine if vaccination against influenza decreases the incidence of influenza, influenza-like illness, or related complications.
The review of children included 41 randomized controlled trials (RCTs) with more than 200,000 patients between two and 16 years of age, plus 12 case-control and 21 cohort studies. The review compared either live attenuated influenza vaccine (LAIV) or inactivated influenza vaccine with placebo.1 Eighteen of the RCTs addressed vaccine effectiveness, whereas the remainder addressed vaccine safety only. Studies were conducted in the United States, Western Europe, Russia, and Bangladesh between 1984 and 2013, mostly over single influenza seasons. Although individual comparisons of LAIV or inactivated influenza vaccine against placebo in various age groups led to different estimates of absolute and relative effectiveness, the small number of studies used for each comparison precluded definitive age-stratified conclusions on vaccine effectiveness. The reviewers instead reported overall effectiveness estimates for LAIV and inactivated influenza vaccine stratified in relation to estimated baseline rates for low-, moderate-, and high-risk groups because of wide variation in event rates across the included studies.
The reviewers found that in a moderate-risk population of children, vaccination of seven children with LAIV or five children with inactivated influenza vaccine would prevent one case of laboratory-confirmed influenza in a season. However, in the same population, vaccination of 19 children with LAIV or 13 children with inactivated influenza vaccine would be necessary to prevent one episode of influenza-like illness. The reviewers found inconsistent evidence suggesting that the incidence of otitis media might be decreased by LAIV but increased by inactivated influenza vaccine. The estimates of effect size for prevention of otitis media were too broad to allow any firm conclusion of risk or benefit. Similarly, the estimates of effect size for reducing the number of days children missed school (LAIV and inactivated influenza vaccine) were too broad to provide any firm conclusions regarding risk or benefit. No information in the reviews addressed the impact of LAIV or inactivated influenza vaccine on influenza-related deaths in children. The results suggested LAIV might be more effective with a two-dose schedule compared with a one-dose schedule. All studies of inactivated influenza vaccine used single-dose schedules. Approximately one-half of the RCTs addressing vaccine efficacy or effectiveness were at low risk of bias, whereas the remainder were at high or uncertain risk of bias related to randomization, allocation, blinding, or missing data.
The review of adult vaccination studies identified 52 clinical trials of more than 80,000 healthy people 16 to 65 years of age and reported data from 25 RCTs comparing inactivated parenteral influenza vaccine and placebo.2 Studies were conducted in North America, South America, and Europe between 1969 and 2009, mostly over single influenza seasons. The reviewers found that vaccination of 71 adults would prevent one case of laboratory-confirmed influenza in a season. Because baseline risk of influenza-like illness varied significantly across studies, from 0.6% (low) to 3.4% (moderate) to 14.6% (high), the authors combined estimates of absolute and relative effectiveness and reported summary estimates in relation to all three levels of baseline risk. For preventing influenza-like illness, the reviewers found that in a moderate-risk population, vaccination of 29 adults would prevent one episode of influenza-like illness in a season. The ranges of effect size estimates for prevention of hospitalization and prevention of nausea or vomiting were too broad to allow any firm conclusions to be drawn. The studies also suggested that for every 125 adults vaccinated, one additional person might experience a fever. Sixteen percent of the data sets were at low risk of bias, whereas the remainder of the data sets were at high risk of bias related to randomization, allocation, blinding, or missing data.
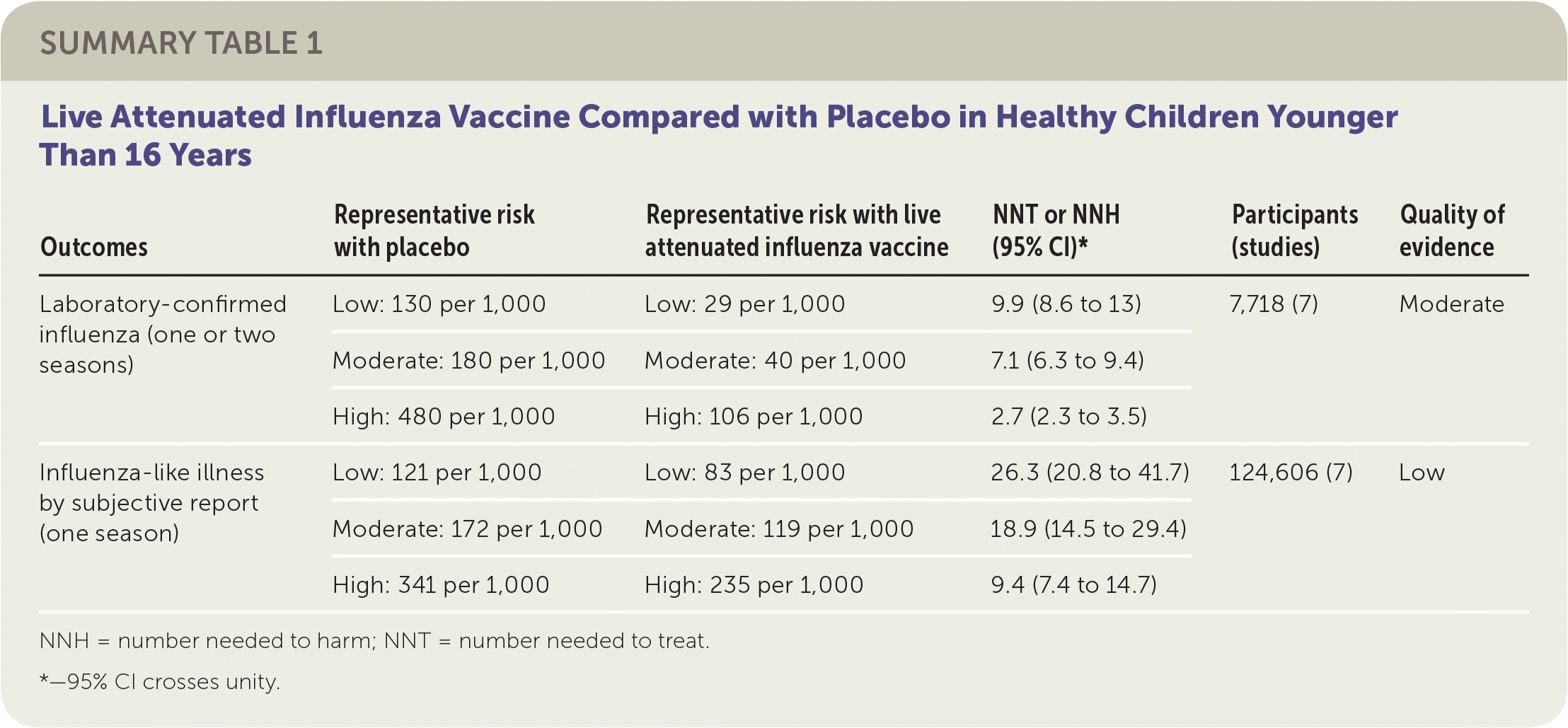
| Outcomes | Representative risk with placebo | Representative risk with live attenuated influenza vaccine | NNT or NNH (95% CI)* | Participants (studies) | Quality of evidence |
|---|---|---|---|---|---|
| Laboratory-confirmed influenza (one or two seasons) | Low: 130 per 1,000 | Low: 29 per 1,000 | 9.9 (8.6 to 13) | 7,718 (7) | Moderate |
| Moderate: 180 per 1,000 | Moderate: 40 per 1,000 | 7.1 (6.3 to 9.4) | |||
| High: 480 per 1,000 | High: 106 per 1,000 | 2.7 (2.3 to 3.5) | |||
| Influenza-like illness by subjective report (one season) | Low: 121 per 1,000 | Low: 83 per 1,000 | 26.3 (20.8 to 41.7) | 124,606 (7) | Low |
| Moderate: 172 per 1,000 | Moderate: 119 per 1,000 | 18.9 (14.5 to 29.4) | |||
| High: 341 per 1,000 | High: 235 per 1,000 | 9.4 (7.4 to 14.7) |
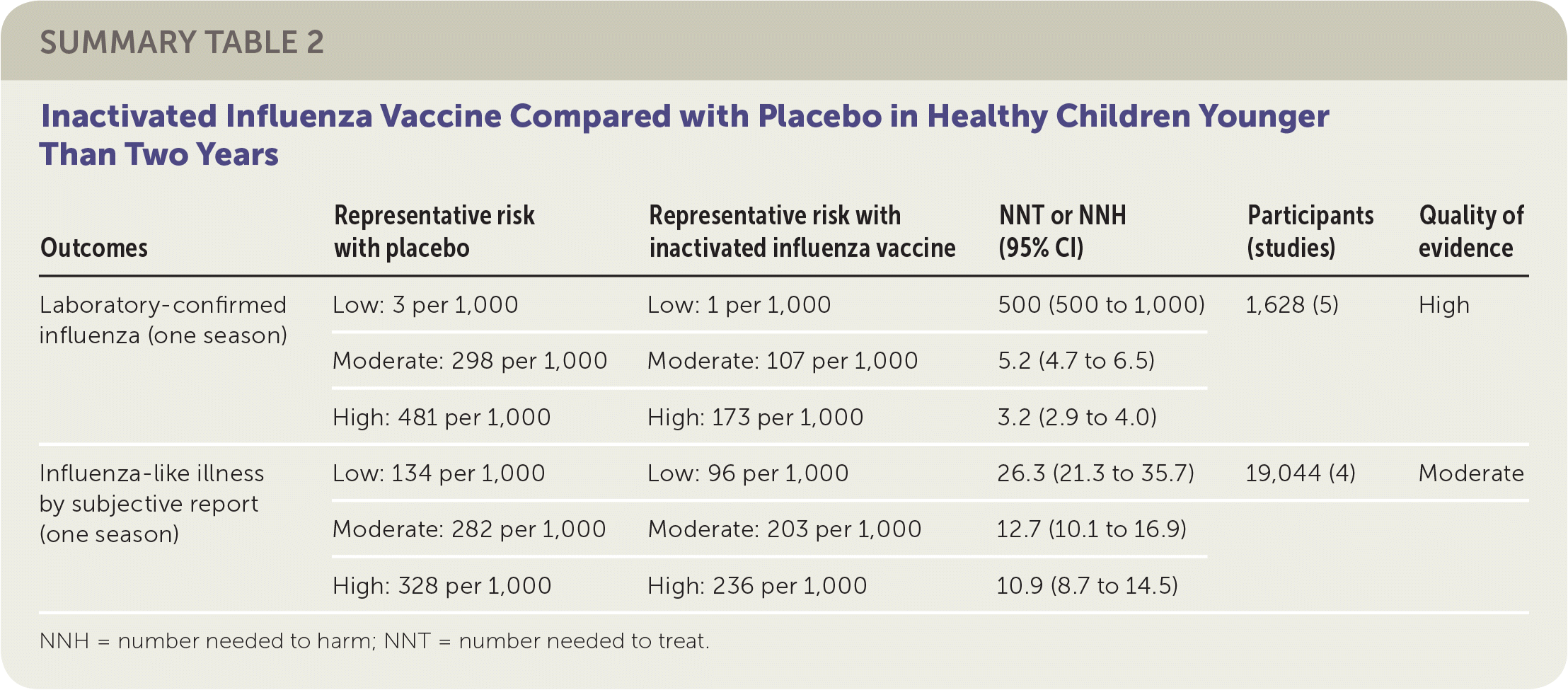
| Outcomes | Representative risk with placebo | Representative risk with inactivated influenza vaccine | NNT or NNH (95% CI) | Participants (studies) | Quality of evidence |
|---|---|---|---|---|---|
| Laboratory-confirmed influenza (one season) | Low: 3 per 1,000 | Low: 1 per 1,000 | 500 (500 to 1,000) | 1,628 (5) | High |
| Moderate: 298 per 1,000 | Moderate: 107 per 1,000 | 5.2 (4.7 to 6.5) | |||
| High: 481 per 1,000 | High: 173 per 1,000 | 3.2 (2.9 to 4.0) | |||
| Influenza-like illness by subjective report (one season) | Low: 134 per 1,000 | Low: 96 per 1,000 | 26.3 (21.3 to 35.7) | 19,044 (4) | Moderate |
| Moderate: 282 per 1,000 | Moderate: 203 per 1,000 | 12.7 (10.1 to 16.9) | |||
| High: 328 per 1,000 | High: 236 per 1,000 | 10.9 (8.7 to 14.5) |
The review of older adult vaccination studies included eight RCTs and 5,000 participants older than 65 years comparing influenza vaccines with placebo.3 Studies were conducted in the United States and Europe between 1965 and 2000 in both community and residential care settings. The reviewers found that vaccination of 30 older adults would prevent one case of laboratory-confirmed influenza in a season, and 42 older adults would need to be vaccinated to prevent one episode of influenza-like illness in a season. The included studies did not demonstrate any effect of influenza vaccination on mortality. The studies in this review were at high or uncertain risk of bias because of publication bias, as well as issues related to blinding and missing data.
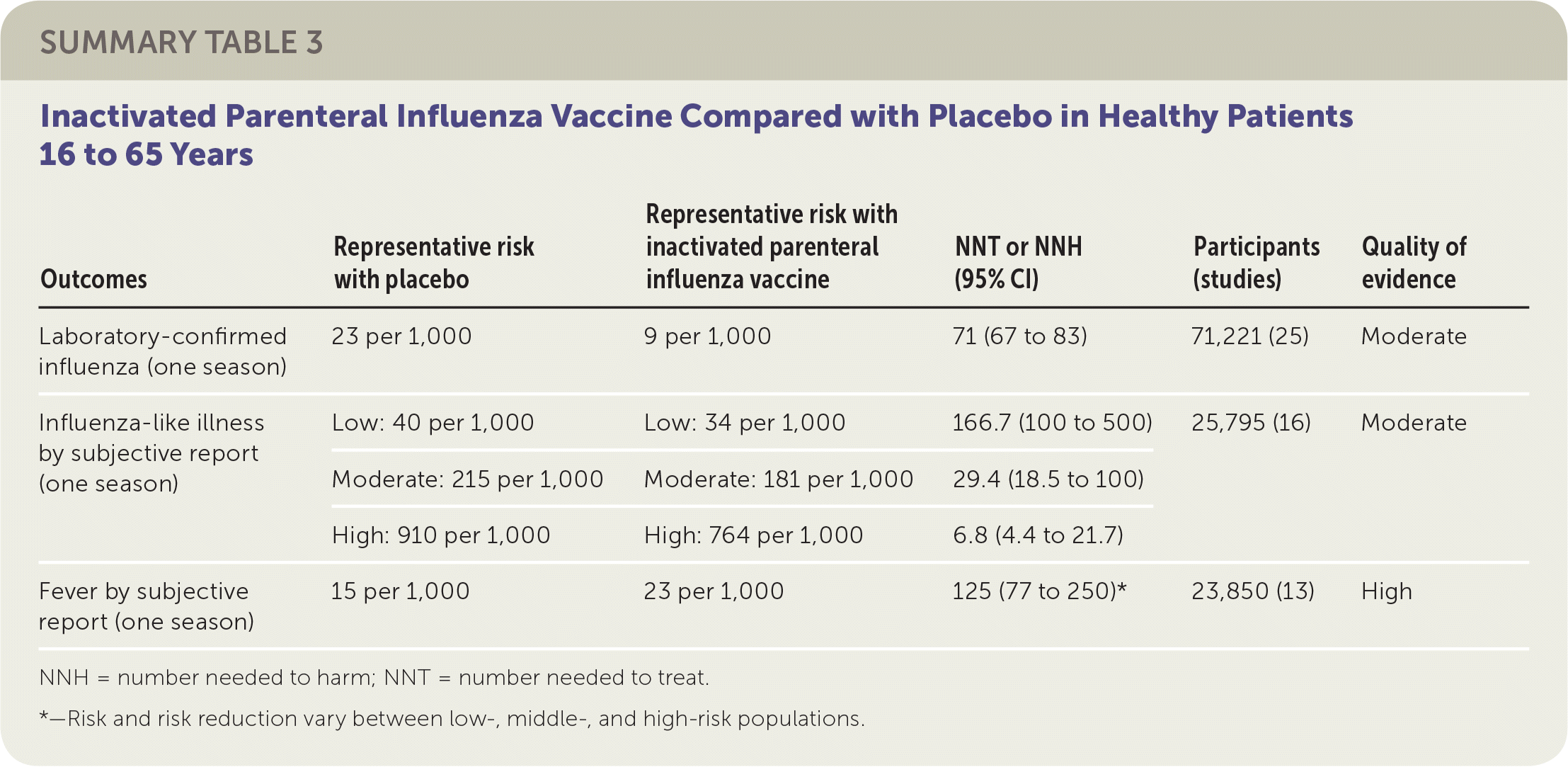
| Outcomes | Representative risk with placebo | Representative risk with inactivated parenteral influenza vaccine | NNT or NNH (95% CI) | Participants (studies) | Quality of evidence |
|---|---|---|---|---|---|
| Laboratory-confirmed influenza (one season) | 23 per 1,000 | 9 per 1,000 | 71 (67 to 83) | 71,221 (25) | Moderate |
| Influenza-like illness by subjective report (one season) | Low: 40 per 1,000 | Low: 34 per 1,000 | 166.7 (100 to 500) | 25,795 (16) | Moderate |
| Moderate: 215 per 1,000 | Moderate: 181 per 1,000 | 29.4 (18.5 to 100) | |||
| High: 910 per 1,000 | High: 764 per 1,000 | 6.8 (4.4 to 21.7) | |||
| Fever by subjective report (one season) | 15 per 1,000 | 23 per 1,000 | 125 (77 to 250)* | 23,850 (13) | High |
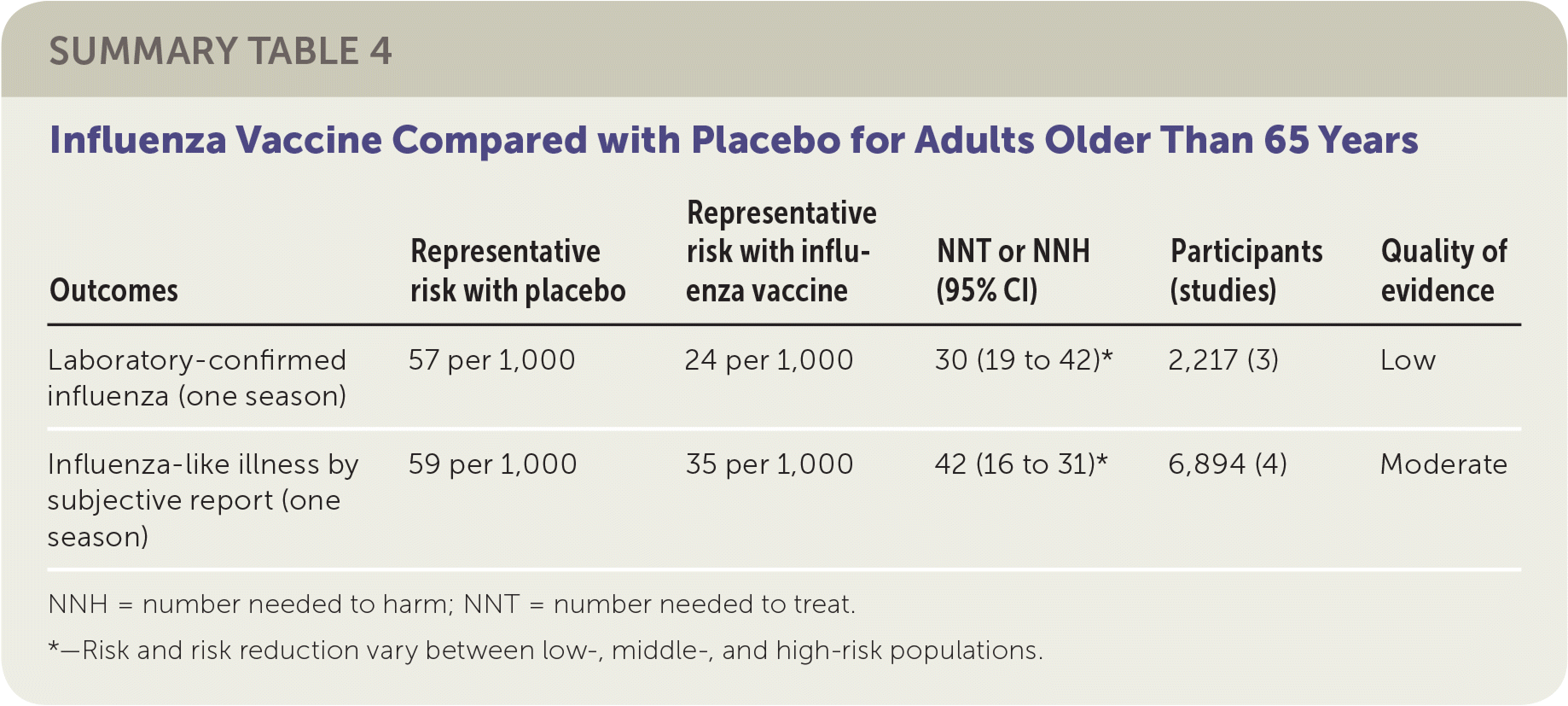
| Outcomes | Representative risk with placebo | Representative risk with influenza vaccine | NNT or NNH (95% CI) | Participants (studies) | Quality of evidence |
|---|---|---|---|---|---|
| Laboratory-confirmed influenza (one season) | 57 per 1,000 | 24 per 1,000 | 30 (19 to 42)* | 2,217 (3) | Low |
| Influenza-like illness by subjective report (one season) | 59 per 1,000 | 35 per 1,000 | 42 (16 to 31)* | 6,894 (4) | Moderate |
The authors of these three Cochrane reviews have been regularly updating the evidence on influenza vaccine effectiveness for more than 20 years, and they do not plan further updates to these reviews until (1) there is a new trial that meets inclusion criteria, (2) there is a new generation of influenza vaccines, or (3) there is development of a new causal paradigm for influenza-like illness.6
The Centers for Disease Control and Prevention advises annual influenza vaccination for all persons older than six months who do not have contraindications to vaccination as a way to avoid getting influenza and to reduce the risks of influenza-like illnesses, hospitalizations, and deaths from influenza among children.7
The practice recommendations in this activity are available at http://www.cochrane.org/CD004879, http://www.cochrane.org/CD001269, and http://www.cochrane.org/CD004876.
Editor's Note: Some of the numbers needed to treat and harm reported in this Cochrane for Clinicians were calculated by the author based on raw data provided in the original Cochrane review.
