
Am Fam Physician. 2020;101(6):339-340
Author disclosure: No relevant financial affiliations.
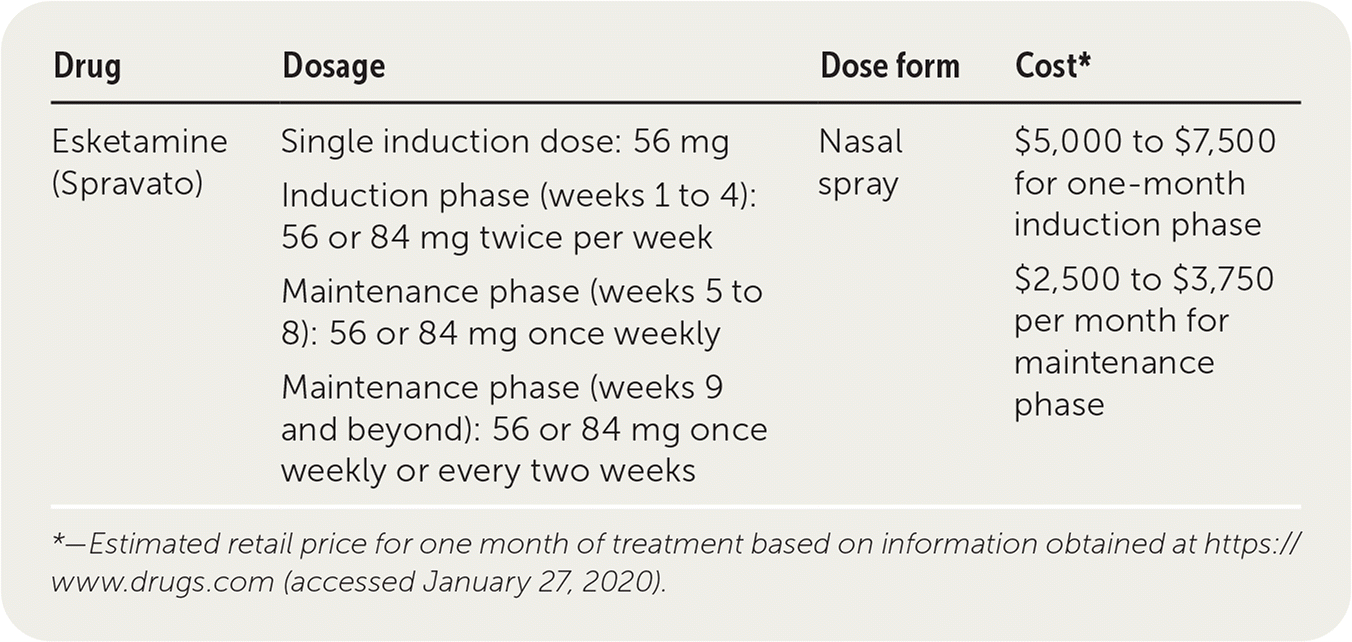
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Esketamine (Spravato) | Single induction dose: 56 mg Induction phase (weeks 1 to 4): 56 or 84 mg twice per week Maintenance phase (weeks 5 to 8): 56 or 84 mg once weekly Maintenance phase (weeks 9 and beyond): 56 or 84 mg once weekly or every two weeks | Nasal spray | $5,000 to $7,500 for one-month induction phase $2,500 to $3,750 per month for maintenance phase |
Safety
Dissociation, in which a patient detaches from or becomes less aware of his or her surroundings, will occur in about 40% of patients who receive esketamine. More than one-half of patients will also experience sedation.1 Patients should be carefully observed for at least two hours after administration of esketamine and should not drive until the following day. The potential for abuse or diversion while taking esketamine is high, and the manufacturer suggests that caution be used in patients with a history of drug abuse or dependence. Esketamine should not be used in patients with a history of aneurysmal vascular disease, arteriovenous malformations, or intracerebral hemorrhage because it may cause a significant increase in blood pressure or intracranial pressure. Patients should have their blood pressure measured before and after administration. Esketamine should not be used in patients with severe hepatic impairment or in children or adolescents because these groups have not been studied.1,2 Esketamine has been shown to impair neuronal development when administered to pregnant or nursing animals, and it should not be used in pregnant or breastfeeding women.1
Tolerability
Adverse effects are common with esketamine. Dizziness, nausea, and vertigo occur in 20% to 30% of patients. Some will have hypoesthesia (18%) or anxiety (13%). Up to 17% will experience an increase of more than 40 mm Hg in systolic blood pressure and 25 mm Hg in diastolic blood pressure in the first 40 to 90 minutes after administration. The risk of an increase in blood pressure is higher in patients who are also taking monoamine oxidase inhibitors or stimulants. Patients should be referred for immediate emergency care if they experience symptoms of hypertensive crisis (e.g., chest pain, shortness of breath) or hypertensive encephalopathy (e.g., sudden severe headache, visual disturbances) after administration of esketamine.1 About 5% of patients will discontinue treatment because of adverse effects.
Effectiveness
Esketamine has been studied in two randomized, placebo-controlled, double-blind studies of 565 patients with a severe major depressive disorder whose current depressive symptoms were not responding to at least two different adequately dosed antidepressants. The addition of esketamine lowered the Montgomery-Åsberg Depression Rating Scale (MADRS) score by 3.8 more points (95% CI, −6.3 to −1.4) out of a possible 60 points compared with placebo after at least four weeks of treatment.3 This difference was statistically but not clinically significant.3,4 Although significantly more patients in the esketamine group experienced a response to treatment, defined as a greater than 50% MADRS score reduction (53% vs. 42%; number needed to treat [NNT] = 9), there was no significant difference in patients attaining clinical remission (defined as a MADRS total score of 12 or less for at least three weeks) compared with placebo. In a study of 137 patients 65 years and older, the addition of esketamine did not have a significant effect in lowering depression scores.3 Esketamine, as an adjunct to oral antidepressant therapy, has not been compared with other adjunctive therapies.
Continuous treatment with esketamine has maintained remission or response in 297 adults.5 After an average of 10.1 to 19.4 additional weeks of exposure to esketamine or placebo, significantly fewer patients in remission relapsed while receiving esketamine compared with those receiving placebo (25.8% vs. 45.3%; NNT = 5; 95% CI, 4 to 18). Of the patients who initially responded to treatment but did not achieve remission, 26.7% of those treated with esketamine experienced relapse compared with 57.6% of patients treated with placebo (NNT = 4; 95% CI, 2 to 5). Patients also had a longer time until depression relapse when treated with esketamine vs. placebo (153 days vs. 33 days; P = .003).
Price
The one-month induction phase of esketamine therapy ranges from about $5,000 for the 56-mg dose to $7,500 for the 84-mg dose. The monthly cost for the once-weekly maintenance dosage ranges from $2,500 (56 mg) to $3,750 (84 mg). Esketamine is significantly more expensive than using oral antidepressants alone or with augmentation.6 The Institute for Clinical and Economic Review suggests that payers develop prior authorization criteria because the long-term clinical benefit has not yet been demonstrated.3
Simplicity
Esketamine requires a prescription and must be administered under the direct supervision of a health care professional. Only clinicians enrolled in the manufacturer's Risk Evaluation and Mitigation Strategies (REMS) can prescribe and administer esketamine.1,2 The initial dose of esketamine is 56 mg taken intranasally, and subsequent 56-mg or 84-mg dosages should be administered twice per week for the next four weeks based on the discretion of the REMS clinician. The higher 84-mg dosage should be considered for patients in whom at least three or more antidepressants have failed.2 If these initial dosages are tolerated, they should continue to be given once weekly for the next four weeks. After week 9, patients can continue to receive the 54-mg or 84-mg dosage every week or every other week.
Bottom Line
Esketamine is an in-office treatment for patients with a severe major depressive disorder to be used in addition to oral antidepressant therapy. Because of its high cost, major safety concerns, and requirements for patient monitoring, esketamine should be considered only in patients who are unable to receive or tolerate electroconvulsive therapy or intravenous ketamine.
