
Am Fam Physician. 2020;102(9):567-570
Author disclosure: No relevant financial affiliations.
Case Scenario
A 50-year-old patient, M.W., successfully reached their goal weight by losing 15 lb (6.8 kg) over six months on a Mediterranean diet. After maintaining the weight loss for more than a year, my patient is now 30 lb (13.6 kg) over their ideal body weight of 140 lb (63.5 kg). My patient has developed cardiometabolic syndrome and receives intensive lifestyle counseling. M.W. feels ashamed by the “failure” and has been avoiding seeing a physician.
Commentary
Internalized weight bias, or self-criticism of one's weight, can cause significant distress in patients trying to lose weight1 and may be particularly pronounced in patients who have regained some or most of their original weight loss.2 An appropriate clinical response to a patient's weight regain includes validation of the patient's effort, acknowledgment of the biological set point that regulates weight as tightly as sodium or water balance,3 and a step-wise approach to weight loss that incorporates lifestyle changes, environmental factors, pharmacotherapy, and, if necessary, bariatric surgery.
To assist a patient who has experienced weight regain, the physician should acknowledge, congratulate, and build on the patient's previous commitment and success in losing weight.4 The patient should be reminded that weight regain may not be a failure of willpower but rather part of the body's innate biology to maintain weight for survival.3 Following weight loss, this drive causes an increase in appetite and produces adaptive thermogenesis, or a lower metabolic rate, compared with others who have a similar body composition; however, there are various ways to ensure long-term weight-loss success.5
To begin, the definition of successful weight loss should be clarified to the patient. For those at risk of diabetes mellitus, a 5% weight loss has shown a significant decrease in progression to diabetes, whereas a 5% to 10% weight loss is needed to see significant reductions in A1C for people who have confirmed diabetes. For most patients, a 5% weight loss results in a modest improvement in cholesterol and triglyceride levels and blood pressure.6 A 3% to 5% minimum weight loss seems necessary to improve steatosis in patients with nonalcoholic fatty liver disease, whereas a 7% to 10% weight loss is needed to improve fibrosis.7
Optimization of lifestyle with diet and activity plus behavioral support remain critical interventions in weight management after weight regain. These changes may be perceived as less difficult by focusing on the positive impact of a healthy diet rather than on dietary deprivation or extreme physical or psychosocial measures. A simple set of goals, consistent with the literature that describes how lifestyle behaviors support longevity, can be established using the 5-2-1-0 approach8 (Table 18,9).

| 5 | 5 servings of fruits and vegetables per day 5% weight loss if body mass index ≥ 30 kg per m2; consider body mass index ≥ 27 kg per m2 in Asian or Asian American patients9 |
| 2 | 2 hours or less of nonwork screen time per day 2 drinks or less of alcohol per day for men |
| 1 | 1 hour of activity per day 1 drink or less of alcohol per day for women |
| 0 | 0 sweetened drinks No smoking |
If these strategies are ineffective, pharmacotherapy can be an appropriate next step (Table 210–22). To be approved by the U.S. Food and Drug Administration (FDA) for weight loss, a medication must show at least a 5% weight loss vs. a placebo.22 Other than orlistat (Xenical), a pancreatic lipase inhibitor that works in the gastrointestinal tract to inhibit triglyceride absorption, all other anti-obesity–specific medications work through neurohormonal changes that decrease appetite.22 Currently, no medications approved by the FDA increase thermogenesis or energy expenditure. The lack of pharmacologic interventions that can affect the pathways responsible for changing the body's set point explains why medications are only modestly effective in helping maintain weight loss and combating weight regain. On average, anti-obesity medications result in a 5% to 10% weight loss, and patients should have achieved at least a 5% weight loss after three months on the maximally tolerated dose to continue taking an anti-obesity medication. Phentermine is the only weight-loss medication not approved by the FDA for long-term use. A randomized trial demonstrated that a combination of a behavioral weight-loss program and medication is superior to either strategy alone.23
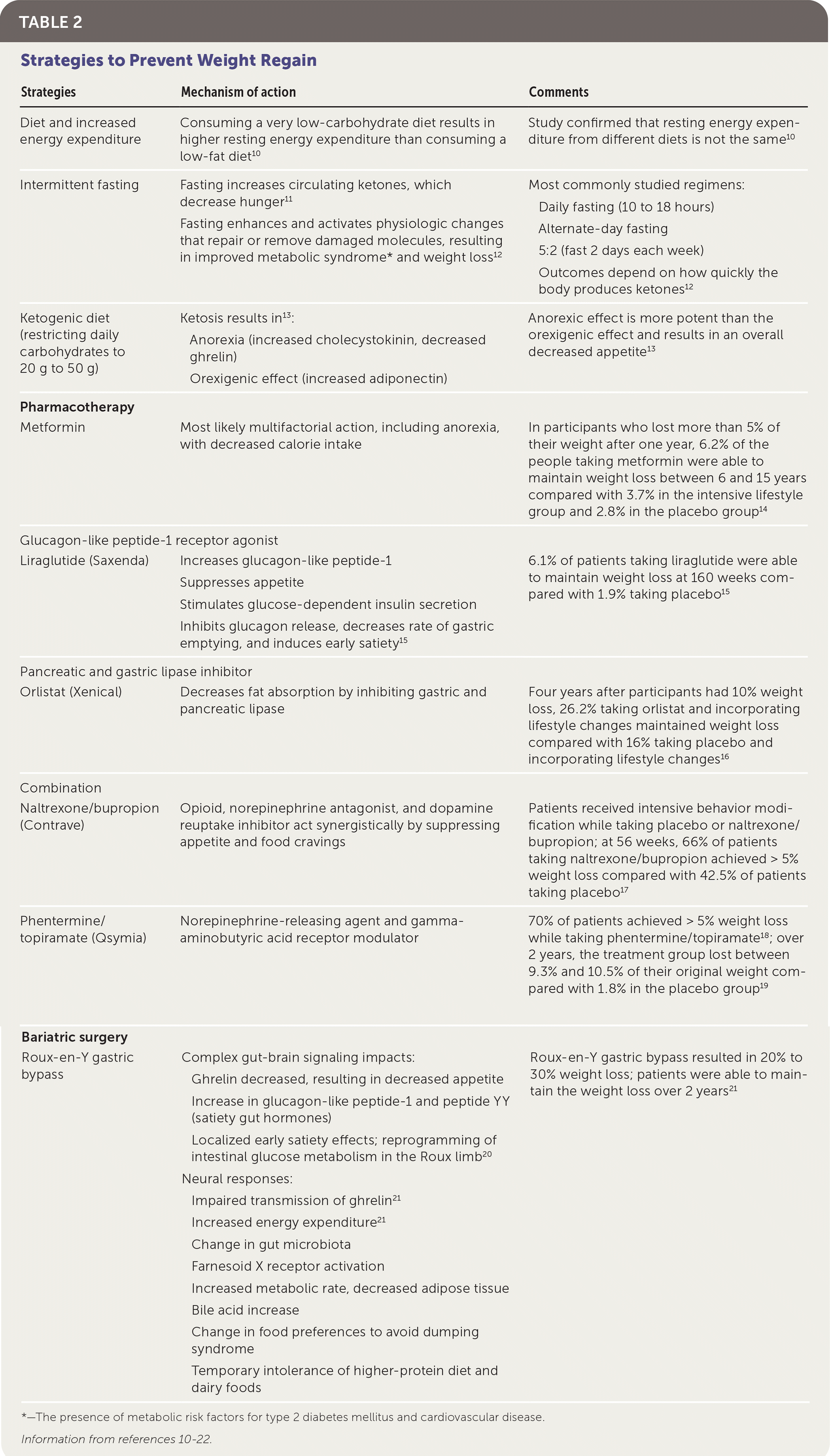
| Strategies | Mechanism of action | Comments |
|---|---|---|
| Diet and increased energy expenditure | Consuming a very low-carbohydrate diet results in higher resting energy expenditure than consuming a low-fat diet10 | Study confirmed that resting energy expenditure from different diets is not the same10 |
| Intermittent fasting | Fasting increases circulating ketones, which decrease hunger11 Fasting enhances and activates physiologic changes that repair or remove damaged molecules, resulting in improved metabolic syndrome* and weight loss12 | Most commonly studied regimens: Daily fasting (10 to 18 hours) Alternate-day fasting 5:2 (fast 2 days each week) Outcomes depend on how quickly the body produces ketones12 |
| Ketogenic diet (restricting daily carbohydrates to 20 g to 50 g) | Ketosis results in13: Anorexia (increased cholecystokinin, decreased ghrelin) Orexigenic effect (increased adiponectin) | Anorexic effect is more potent than the orexigenic effect and results in an overall decreased appetite13 |
| Pharmacotherapy | ||
| Metformin | Most likely multifactorial action, including anorexia, with decreased calorie intake | In participants who lost more than 5% of their weight after one year, 6.2% of the people taking metformin were able to maintain weight loss between 6 and 15 years compared with 3.7% in the intensive lifestyle group and 2.8% in the placebo group14 |
| Glucagon-like peptide-1 receptor agonist | ||
| Liraglutide (Saxenda) | Increases glucagon-like peptide-1 Suppresses appetite Stimulates glucose-dependent insulin secretion Inhibits glucagon release, decreases rate of gastric emptying, and induces early satiety15 | 6.1% of patients taking liraglutide were able to maintain weight loss at 160 weeks compared with 1.9% taking placebo15 |
| Pancreatic and gastric lipase inhibitor | ||
| Orlistat (Xenical) | Decreases fat absorption by inhibiting gastric and pancreatic lipase | Four years after participants had 10% weight loss, 26.2% taking orlistat and incorporating lifestyle changes maintained weight loss compared with 16% taking placebo and incorporating lifestyle changes16 |
| Combination | ||
| Naltrexone/bupropion (Contrave) | Opioid, norepinephrine antagonist, and dopamine reuptake inhibitor act synergistically by suppressing appetite and food cravings | Patients received intensive behavior modification while taking placebo or naltrexone/bupropion; at 56 weeks, 66% of patients taking naltrexone/bupropion achieved > 5% weight loss compared with 42.5% of patients taking placebo17 |
| Phentermine/topiramate (Qsymia) | Norepinephrine-releasing agent and gamma-aminobutyric acid receptor modulator | 70% of patients achieved > 5% weight loss while taking phentermine/topiramate18; over 2 years, the treatment group lost between 9.3% and 10.5% of their original weight compared with 1.8% in the placebo group19 |
| Bariatric surgery | ||
| Roux-en-Y gastric bypass | Complex gut-brain signaling impacts: Ghrelin decreased, resulting in decreased appetite Increase in glucagon-like peptide-1 and peptide YY (satiety gut hormones) Localized early satiety effects; reprogramming of intestinal glucose metabolism in the Roux limb20 Neural responses: Impaired transmission of ghrelin21 Increased energy expenditure21 Change in gut microbiota Farnesoid X receptor activation Increased metabolic rate, decreased adipose tissue Bile acid increase Change in food preferences to avoid dumping syndrome Temporary intolerance of higher-protein diet and dairy foods | Roux-en-Y gastric bypass resulted in 20% to 30% weight loss; patients were able to maintain the weight loss over 2 years21 |
When lifestyle and behavior modifications paired with pharmacotherapy fail to help a patient achieve weight-loss goals, bariatric surgery is the most effective intervention for weight loss, weight maintenance, and prevention of weight regain. The reason that bariatric surgery results in a longer-term adjustment in the set point is not entirely understood; however, postsurgical changes in the gastrointestinal hormones glucagon-like peptide-1, peptide YY, and ghrelin likely play significant roles.24 Bariatric surgery is the only current weight-loss intervention that reproducibly results in diabetes remission,25 which in turn could result in subsequent reductions in cancer, cardiovascular disease, and premature mortality.26
In the case scenario, the physician should first recognize and then praise M.W.'s previous accomplishment in weight loss and dedication to attending intensive lifestyle counseling. M.W. should be reassured that although neurobiologic changes can hinder weight-loss success by influencing appetite and altering metabolism, a long-term, multimodal approach can help with achieving and maintaining realistic weight loss. The 5-2-1-0 approach can assist with lifestyle modification8 (Table 18,9). M.W. may further benefit from the addition of pharmacotherapy to support therapeutic lifestyle changes. If M.W. is hypertensive or taking a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor, liraglutide (Saxenda), bupropion (Wellbutrin), and phentermine should be avoided or prescribed only with caution. If M.W. achieves a 5% weight loss after three months with pharmacotherapy and has negligible adverse effects, long-term therapy is appropriate. Because lifestyle changes or pharmacotherapy is unlikely to help a patient achieve a weight loss greater than 10%, a discussion about bariatric surgery is appropriate if the risks of excess weight outweigh the risks of the current bariatric options27 (Table 210–22).
