
Am Fam Physician. 2020;102(9):627-628
Author disclosure: No relevant financial affiliations.
Oral semaglutide (Rybelsus) is a glucagon-like peptide-1 (GLP-1) receptor agonist labeled as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. It reduces hyperglycemia by increasing insulin secretion and decreasing glucagon secretion.
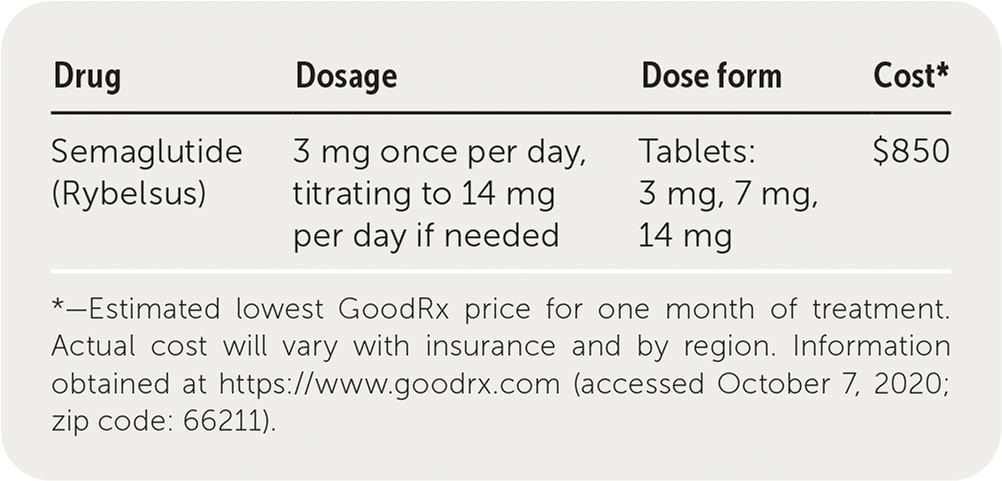
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Semaglutide (Rybelsus) | 3 mg once per day, titrating to 14 mg per day if needed | Tablets: 3 mg, 7 mg, 14 mg | $850 |
Safety
Similar to other medications in this class, semaglutide carries the risk of an increased incidence of thyroid C-cell tumors, including medullary thyroid carcinoma.1 Patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2 should not be prescribed oral semaglutide. Serious adverse effects are similar to those of other GLP-1 receptor agonists, including pancreatitis, diabetic retinopathy complications, hypoglycemia with concomitant use of insulin secretagogues or insulin, acute kidney injury, and hypersensitivity reactions (less than 1% to 3%).1 Oral semaglutide has not been studied in pregnant patients or breastfed infants.1
Tolerability
Approximately 30% to 50% of patients taking oral semaglutide will experience gastrointestinal adverse effects. Nausea, vomiting, diarrhea, dyspepsia, and constipation are the most common. Abdominal pain, eructation, gastroesophageal reflux disease, and gastritis have been reported but are less common.2 In clinical trials, the dropout rate due to adverse effects is 8% to 16%.2
Effectiveness
Oral semaglutide improves glycemic control in adults with type 2 diabetes better than placebo and is noninferior to subcutaneous semaglutide.2 Although oral semaglutide is marketed in the United States as 3-mg, 7-mg, and 14-mg tablets, a 26-week randomized, parallel group clinical trial spanning 14 countries compared daily oral 2.5-mg, 5-mg, 10-mg, 20-mg, and 40-mg semaglutide with placebo and weekly 1-mg subcutaneous semaglutide (Ozempic). The study enrolled 632 adults with type 2 diabetes and a mean age of 57 years, mean A1C of 7.9%, and body mass index of 31.7 kg per m2. Across the oral doses of 2.5 mg, 5 mg, 10 mg, and 20 mg, oral semaglutide lowered mean A1C by 0.7%, 1.2%, 1.5%, and 1.7%, respectively, compared with a decrease of 1.9% with subcutaneous semaglutide. In these patients with mildly elevated baseline A1C levels (7.8% to 8.0%), all oral doses except 2.5 mg reduced A1C to less than 7.0% in more than 80% of those treated. The 10-mg daily dosage resulted in an average weight loss of 10.6 lb (4.8 kg) over 26 weeks (95% CI, 8.2 to 12.8 lb [3.7 to 5.8 kg]), which is similar to the average weight loss of 14.1 lb (6.4 kg) with 1-mg weekly subcutaneous dosing (95% CI, 11.7 to 16.5 lb [5.3 to 7.5 kg]).
In one short-term study that included mostly male patients 50 years and older with type 2 diabetes and cardiovascular (CV) disease or chronic kidney disease, oral semaglutide did not increase the risk of CV events in high-risk patients, and it was associated with a modest delay of mortality.3
Price
Oral semaglutide costs approximately $850 for a 30-day supply, which is similar to other GLP-1 receptor agonists. However, it is more expensive than other oral diabetic therapies such as empagliflozin (Jardiance) and metformin, which cost about $504 and $5 for one-month supplies, respectively.4,5 Semaglutide may not be covered by all insurance plans.
Simplicity
The initial dosage of semaglutide is 3 mg per day, titrated to desired effect on A1C up to a maximum dosage of 14 mg per day if needed. To ensure adequate absorption, oral semaglutide should be taken 30 minutes before the first meal or drink of the day.
Bottom Line
Oral semaglutide may be considered as an option for patients who need better glycemic control and are unable or reluctant to start or add injectable treatment and for whom weight loss is desired. It does not increase major CV events (including nonfatal stroke or nonfatal myocardial infarction) in higher-risk patients. The cost and hyperglycemia-lowering benefits of oral semaglutide are similar to those of injectable semaglutide; however, it is much more expensive than other oral agents.
