
Am Fam Physician. 2021;103(1):18-19
Clinical Question
In patients with distal (below the knee) deep venous thrombosis (DVT), do vitamin K antagonists safely reduce the risk of recurrence or progression of venous thromboembolism (VTE), and what duration of anticoagulation is best?
Evidence-Based Answer
Vitamin K antagonists reduce the recurrence of DVT and VTE (number needed to treat [NNT] = 17; 95% CI, 13 to 48) but not pulmonary embolism (PE) compared with no anticoagulation or placebo. The risk of clinically relevant nonmajor bleeding (number needed to harm [NNH] = 23; 95% CI, 6 to 1,000), but not major bleeding, is increased with anticoagulation. (Strength of Recommendation [SOR]: A, based on consistent, good-quality patient-oriented evidence.)
A treatment duration of three months or more reduces the recurrence of DVT and VTE (NNT = 13; 95% CI, 10 to 23) compared with six weeks of therapy.1 (SOR: A, based on consistent, good-quality patient-oriented evidence.)
Practice Pointers
The term VTE encompasses a spectrum of disease ranging from mild calf symptoms from DVT to life-threatening cardiopulmonary collapse from PE. Isolated distal DVT is a subset of VTE with decreased risk of recurrent DVT and PE, compared with more proximal disease.2 A strategy of close observation may be appropriate in those with isolated distal DVT given the lower risk of complications. The authors of this review searched for randomized controlled trials (RCTs) addressing the effectiveness and safety of treatments for distal DVT, as well as those comparing varying durations of anticoagulation.
This Cochrane review included data from eight RCTs and 1,239 participants.1 Trials included patients with provoked and unprovoked DVT, although patients with cancer (five of eight studies) and prior VTE (six of eight studies) were often excluded. All but one trial were open label, raising the possibility of performance bias, although the authors deemed the reported outcomes unlikely to be affected. Six of the trials used vitamin K antagonists as the active treatment agent, and two used the low-molecular-weight heparin nadroparin (not available in the United States). Trials using vitamin K antagonists had a lead-in period with a parenteral agent during initiation, and follow-up ranged from three months to two years. Five of the trials (496 participants) compared anticoagulants of varying durations with no treatment or placebo. The other three studies (736 participants) compared patients treated with six weeks of anticoagulation vs. three months or more. Two studies of nadroparin had a treatment duration of six weeks or less, and outcomes were insufficient to draw conclusions about effectiveness. The authors did not find completed studies that reported on the use of compression therapy or direct oral anticoagulants (DOACs) specifically for distal DVT.
Patients treated with anticoagulation had decreased rates of recurrent DVT (absolute risk reduction [ARR] = 5.9%; 95% CI, 2.6% to 7.1%; NNT = 17; 95% CI, 14 to 38) and VTE (ARR = 6.0%; 95% CI, 2.1% to 7.7%; NNT = 17; 95% CI, 13 to 48) compared with placebo, but there was no difference in the risk of PE. Rates of major bleeding were not significantly different between the two groups, although rates of clinically significant nonmajor bleeding were higher in the treatment group (absolute risk increase = 4.3%; 95% CI, 0.1% to 17.1%; NNH = 23; 95% CI, 6 to 1,000). Overall incidence of PE and major bleeding was low in both groups.
In studies comparing anticoagulation regimens, patients receiving three months or more of therapy had reduced rates of recurrent DVT (ARR = 9.8%; 95% CI, 5.2% to 12.1%; NNT = 10; 95% CI, 8 to 19) and VTE (ARR = 7.8%; 95% CI, 4.3% to 10%; NNT = 13; 95% CI, 10 to 23) and no difference in bleeding rates compared with patients receiving only six weeks of therapy. Bleeding rates were low in both groups.
The American College of Chest Physicians endorses a strategy of observation with serial ultrasonography to evaluate for clot progression in select patients with distal DVT and no risk factors. Anticoagulation is recommended in the case of severe pain or risk factors, such as an elevated d-dimer level, active cancer, and history of VTE, or for unprovoked clots. These same guidelines favor use of a DOAC over vitamin K antagonists and low-molecular-weight heparin in keeping with the more robust data available for proximal DVT.3 DOACs are associated with similar reductions in VTE and have lower bleeding rates compared with vitamin K antagonists in proximal disease.4 RCTs investigating the DOACs apixaban (Eliquis) and rivaroxaban (Xarelto) for distal DVT are currently underway.1 This review establishes that vitamin K antagonists reduce recurrent VTE after isolated distal DVT without increasing major bleeding events. It also confirms current guidelines that recommend three months of anticoagulation over shorter durations.3
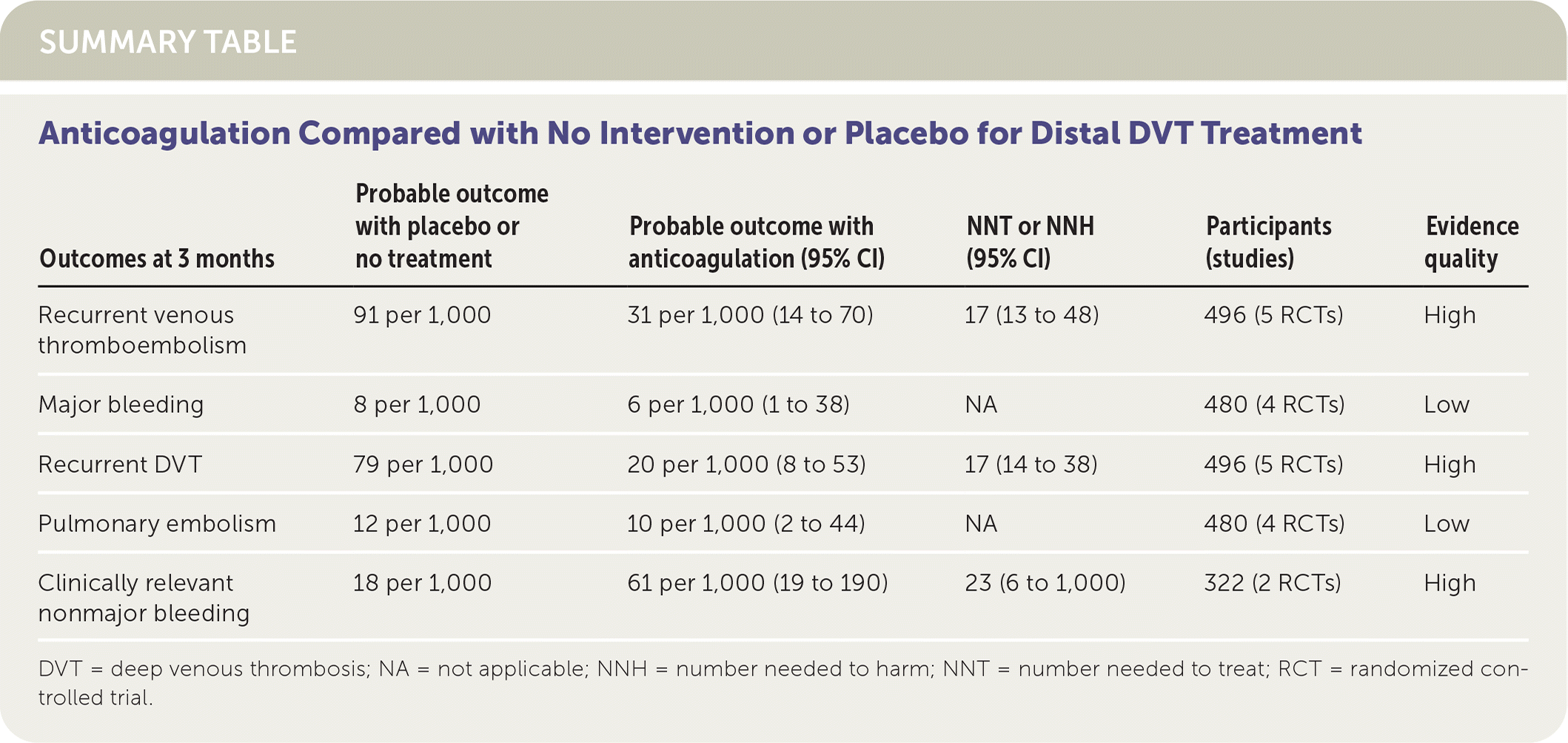
| Outcomes at 3 months | Probable outcome with placebo or no treatment | Probable outcome with anticoagulation (95% CI) | NNT or NNH (95% CI) | Participants (studies) | Evidence quality |
|---|---|---|---|---|---|
| Recurrent venous thromboembolism | 91 per 1,000 | 31 per 1,000 (14 to 70) | 17 (13 to 48) | 496 (5 RCTs) | High |
| Major bleeding | 8 per 1,000 | 6 per 1,000 (1 to 38) | NA | 480 (4 RCTs) | Low |
| Recurrent DVT | 79 per 1,000 | 20 per 1,000 (8 to 53) | 17 (14 to 38) | 496 (5 RCTs) | High |
| Pulmonary embolism | 12 per 1,000 | 10 per 1,000 (2 to 44) | NA | 480 (4 RCTs) | Low |
| Clinically relevant nonmajor bleeding | 18 per 1,000 | 61 per 1,000 (19 to 190) | 23 (6 to 1,000) | 322 (2 RCTs) | High |
The practice recommendations in this activity are available at http://www.cochrane.org/CD013422.
Editor's Note: The NNTs, NNHs, ARRs, and absolute risk increase reported in this Cochrane for Clinicians were calculated by the author based on raw data provided in the original Cochrane review.
