
Am Fam Physician. 2021;103(3):155-163
Author disclosure: No relevant financial affiliations.
Targeted cancer therapies involve chemotherapeutic agents that attack, directly or indirectly, a specific genetic biomarker found in a given cancer. Targeted oncology includes monoclonal antibodies, small molecule inhibitors, antibody-drug conjugates, and immunotherapy. For example, the monoclonal antibodies trastuzumab and pertuzumab target human epidermal growth factor receptor 2 (HER2) and are used when treating HER2-positive breast cancer. Although targeted oncology has improved survival by years for some incurable cancers such as metastatic breast and lung cancer, as few as 8% of patients with advanced cancer qualify for targeted oncology medications, and even fewer benefit. Other limitations include serious adverse events, illustrated by a 20% to 30% rate of heart attack, stroke, or peripheral vascular events among patients taking ponatinib, which is used in treating chronic myelogenous leukemia. Immune checkpoint inhibitor therapy–related adverse effects such as hypothyroidism are common, and more severe adverse events such as colitis and pneumonitis can be fatal and require immediate intervention. Drug interactions with widely prescribed medications such as antacids and warfarin are common. Additionally, financial toxicities are a problem for patients with cancer who are using costly targeted therapies. Future directions for targeted oncology include tumor-agnostic drugs, which target a given mutation and could be used in treating cancers from multiple organ types. An overview of indications, mechanism of action, and toxicities of targeted cancer therapies is offered here.
Targeted cancer therapy involves testing various types of cancer for genetic biomarkers that can predict the response to chemotherapeutic agents that attack the biomarkers directly or indirectly.1,2 In the past decade, the U.S. Food and Drug Administration (FDA) has approved approximately 40 new targeted therapies for 12 different cancers3–6 (Table 1). Despite this innovation, the percentage of patients with cancer who are eligible for such therapies is small. In 2018, an estimated 8.3% of 610,000 patients with advanced or metastatic cancer were eligible for targeted therapy.7 The number of patients who benefit from these drugs is even smaller and ranges widely, depending on the tumor and drug. Targeted oncology has mainly shown benefit in the metastatic (incurable) setting, with rare success for patients treated with surgery in the local or regional setting.
WHAT'S NEW ON THIS TOPIC
Targeted Cancer Therapies
In the past decade, the U.S. Food and Drug Administration has approved approximately 40 new targeted therapies for 12 different cancers.
Patients with metastatic epidermal growth factor receptor–mutated lung cancer who are treated with osimertinib (Tagrisso) live a median of 39 months, more than double the survival of similar patients who were treated with the first epidermal growth factor receptor inhibitor, erlotinib (Tarceva), between 2007 and 2011.
In 2020, the average patient out-of-pocket cost for a course of oral cancer therapy was $5,663. According to one large analysis, 20% of patients with cancer take less medication than prescribed, 19% only partially fill oral cancer therapy prescriptions, and 24% avoid filling a prescription at all.
| Drugs | Target | Drug type | FDA-approved indication | Toxicities, adverse effects, precautions | Unique monitoring |
|---|---|---|---|---|---|
| Acute myelogenous leukemia | |||||
| Enasidenib (Idhifa), ivosidenib (Tibsovo) | IDH1/2 | Small molecule inhibitors | Newly diagnosed and relapsed/refractory IDH1/2+ acute myelogenous leukemia | Edema, hepatotoxicity, prolonged QTc | Alkaline phosphatase, ALT, AST, CBC, chemistry, CK, ECG, total bilirubin |
| Gilteritinib (Xospata), midostaurin (Rydapt) | FLT3 | Small molecule inhibitors | Newly diagnosed and relapsed/refractory FLT3+ acute myelogenous leukemia | Hepatotoxicity, prolonged QTc, rash, vomiting | Alkaline phosphatase, ALT, AST, CBC, chemistry, ECG, total bilirubin |
| Anaplastic thyroid cancer | |||||
| Dabrafenib (Tafinlar) plus trametinib (Mekinist) | BRAF and MEK | Small molecule inhibitors | Locally advanced or metastatic with V600E mutation | Colitis, cutaneous squamous cell cancers, fever, heart failure, hepatotoxicity, hyperglycemia, rash, thrombosis | Alkaline phosphatase, ALT, AST, blood glucose, ECG, electrolytes, renal function, skin examination, total bilirubin |
| Bladder cancer | |||||
| Erdafitinib (Balversa) | FGFR2/3 | Small molecule inhibitor | Metastatic or locally advanced FGFR2/3 alterations | Central serous retinopathy, hand-foot syndrome, hyperphosphatemia, oncholysis | Eye examination, phosphate |
| Breast cancer | |||||
| Ado-trastuzumab emtansine (Kadcyla) | HER2 | Antibody-drug conjugate | Early stage HER2+ with residual disease after neoadjuvant treatment; metastatic HER2+ | Cardiotoxicity, hepatotoxicity, interstitial lung disease, neuropathy | Alkaline phosphatase, ALT, AST, CBC, ECG, total bilirubin |
| Alpelisib (Piqray) | PIK3CA | Small molecule inhibitor | PIK3CA-mutated metastatic | Dermatologic (Stevens-Johnson syndrome), hyperglycemia, severe diarrhea | A1C, blood glucose |
| Atezolizumab (Tecentriq) | PD-L1 | Immunotherapy | PD-L1–positive metastatic triple negative breast cancer, in combination with chemotherapy | Colitis, endocrinopathies, hepatitis, myocarditis, pneumonitis, rash | Alkaline phosphatase, ALT, AST, blood glucose, renal function, total bilirubin, TSH |
| Fam-trastuzumab deruxtecan (Enhertu) | HER2 | Antibody-drug conjugate | Metastatic HER2+ | Cardiotoxicity, hematologic, interstitial lung disease (9%) | CBC, echocardiography |
| Olaparib (Lynparza), talazoparib (Talzenna) | Poly- (adenosine diphosphate-ribose) polymerase | Small molecule inhibitors | Breast cancer gene–mutated metastatic | Hematologic, increased mean corpuscular volume, pneumonitis, rare acute myelogenous leukemia | CBC, renal function |
| Pertuzumab (Perjeta) | HER2 | Monoclonal antibody | Metastatic, neoadjuvant, and adjuvant HER2+ | Cardiotoxicity, diarrhea | Echocardiography |
| Chronic lymphocytic leukemia | |||||
| Ibrutinib (Imbruvica) | BTK | Small molecule inhibitor | Chronic lymphocytic leukemia with 17p deletion | Atrial fibrillation, diarrhea, edema, hemorrhage | Alkaline phosphatase, ALT, AST, CBC, renal function, total bilirubin |
| Venetoclax (Venclexta) | BCL2 | Small molecule inhibitor | Chronic lymphocytic leukemia with 17p deletion | Severe pancytopenia, tumor lysis syndrome | CBC, electrolytes, renal function; may require hospitalization for tumor lysis syndrome monitoring |
| Chronic myelogenous leukemia | |||||
| Bosutinib (Bosulif), dasatinib (Sprycel), nilotinib (Tasigna), ponatinib (Iclusig) | BCR-ABL | Small molecule inhibitors | Initial treatment: dasatinib, nilotinib, bosutinib; second-line treatment or T315I mutation: ponatinib | Arterial thrombotic events (ponatinib), diarrhea (bosutinib), edema, effusions (dasatinib), heart failure (all), hematologic, pancreatitis, prolonged QTc (nilotinib) | Alkaline phosphatase, ALT, AST, blood pressure, CBC, chemistry, ECG, glucose, lipid profile, total bilirubin; provide low-dose aspirin with ponatinib |
| Colorectal cancer | |||||
| Cetuximab (Erbitux) | EGFR | Monoclonal antibody | Metastatic without mutation in RAS | Acneiform rash, hypomagnesemia | Electrolytes |
| Gastroesophageal cancer | |||||
| Trastuzumab (Herceptin) | HER2 | Monoclonal antibody | Metastatic with HER2 overexpression | Cardiotoxicity | Echocardiography |
| Gastrointestinal stromal tumor | |||||
| Imatinib (Gleevec) | c-KIT | Small molecule inhibitor | Adjuvant following complete resection of c-KIT positive gastrointestinal stromal tumor | Edema, heart failure, hematologic | Alkaline phosphatase, ALT, AST, CBC, electrolytes, renal function, total bilirubin |
| Lung cancer (adenocarcinoma) | |||||
| Afatinib (Gilotrif), dacomitinib (Vizimpro), erlotinib (Tarceva), gefitinib (Iressa), osimertinib (Tagrisso) | EGFR | Small molecule inhibitors | Metastatic, EGFR exon 19 deletion or exon 21 (L858R) substitution | Diarrhea, hepatotoxicity, prolonged QTc, rash, trichiasis | Alkaline phosphatase, ALT, AST, ECG, electrolytes, renal function, total bilirubin |
| Alectinib (Alecensa), brigatinib (Alunbrig), ceritinib (Zykadia), crizotinib (Xalkori), lorlatinib (Lorbrena) | Anaplastic lymphoma kinase | Small molecule inhibitors | Metastatic, anaplastic lymphoma kinase fusion | Bradycardia, hepatotoxicity, nausea, ocular toxicity, QT prolongation, vomiting | Alkaline phosphatase, ALT, AST, CBC, renal function, total bilirubin |
| Crizotinib, entrectinib (Rozlytrek) | ROS1 | Small molecule inhibitors | Metastatic, ROS1 positive | Entrectinib: cardiotoxicity, cognitive impairment, fractures, hepatotoxicity, ocular toxicity | Alkaline phosphatase, ALT, AST, ECG, echocardiography, electrolytes, total bilirubin |
| Dabrafenib | BRAF | Small molecule inhibitor | Metastatic, BRAF V600E mutation | Cutaneous squamous cell cancer, colitis, fever, heart failure, hepatotoxicity, hyperglycemia, rash, thrombosis | Alkaline phosphatase, ALT, AST, blood glucose, echocardiography, skin examination, total bilirubin |
| Melanoma | |||||
| Binimetinib (Mektovi), cobimetinib (Cotellic), dabrafenib, encorafenib (Braftovi), trametinib, vemurafenib (Zelboraf) | BRAF + MEK | Small molecule inhibitors | Metastatic, V600E, V600K mutation (all) Adjuvant (dabrafenib + trametinib) | BRAF inhibitors: alopecia, arthralgia, diarrhea, fatigue, nausea, rash MEK inhibitors: diarrhea, rash, retinopathy | Alkaline phosphatase, ALT, AST, blood glucose, echocardiography, skin examination, total bilirubin |
| Mismatch repair deficient solid tumors | |||||
| Ipilimumab (Yervoy), nivolumab (Opdivo), pembrolizumab (Keytruda) | PD-1 or CTLA-4 | Immunotherapies | Metastatic mismatch repair deficient solid tumor | Adrenal insufficiency, colitis, myocarditis (rare but morbid), pneumonitis, rash, thyroiditis | Alkaline phosphatase, ALT, AST, blood glucose, renal function, total bilirubin, TSH |
| Neurotrophin receptor kinase fusion solid tumors | |||||
| Entrectinib, larotrectinib (Vitrakvi) | Neurotrophin receptor kinase | Small molecule inhibitors | Metastatic solid tumors with neurotrophin receptor kinase fusion protein | Cardiotoxicity, cognitive impairment, fractures, hepatotoxicity, ocular toxicity | Alkaline phosphatase, ALT, AST, ECG, echocardiography, total bilirubin |
| Ovarian | |||||
| Niraparib (Zejula), olaparib, rucaparib (Rubraca) | Poly- (adenosine diphosphate-ribose) polymerase | Small molecule inhibitors | Advanced or metastatic ovarian cancer with breast cancer gene mutation | Myelodysplastic syndrome, pancytopenia | CBC |
Types of Targeted Therapy
Targeted therapies can be divided into four general categories: monoclonal antibodies, small molecule inhibitors, antibody-drug conjugates, and immunotherapy (Figure 1). In general, small molecule inhibitors are oral, whereas the remaining therapies are given intravenously.
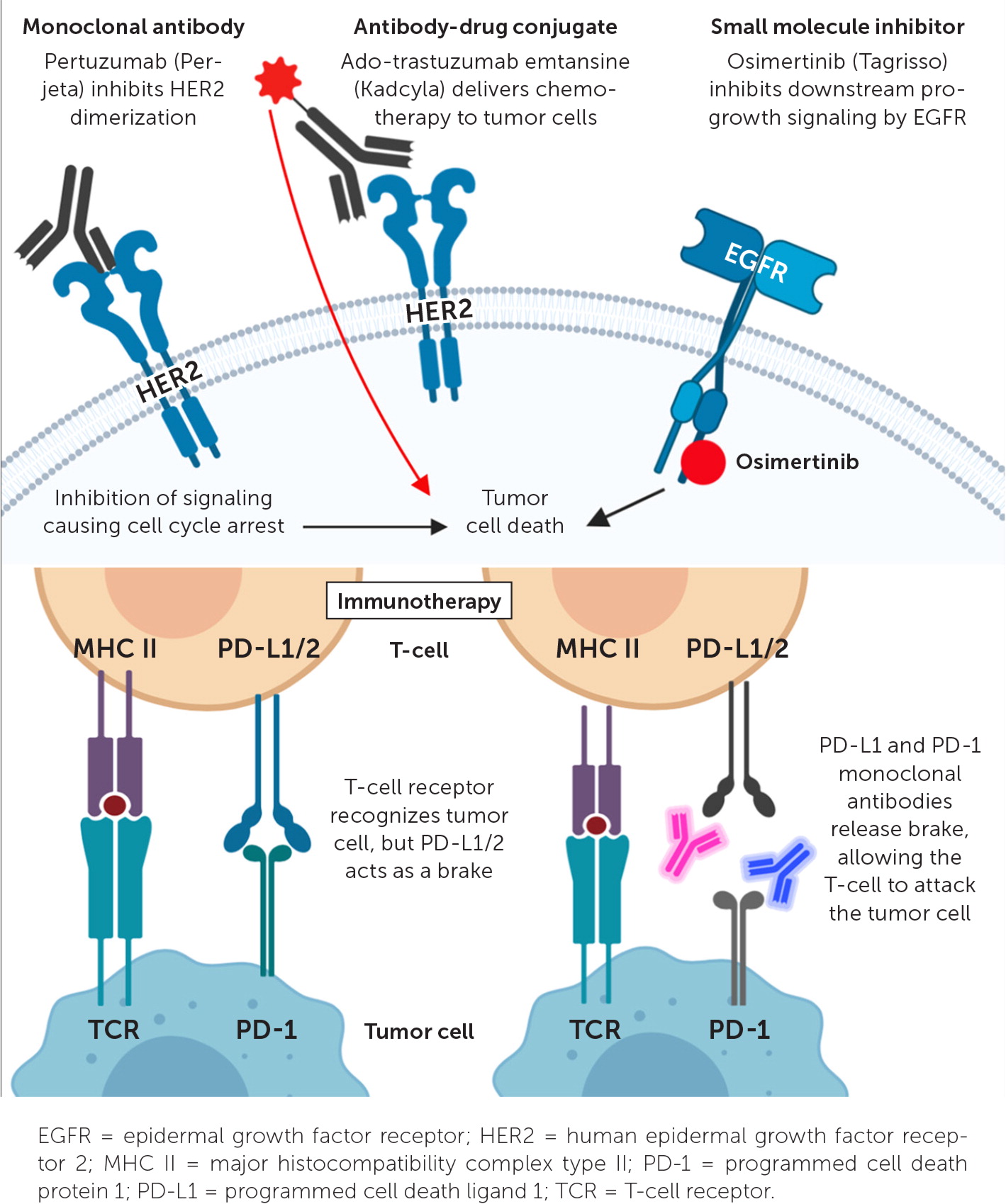
MONOCLONAL ANTIBODIES
Monoclonal antibodies are identical immunoglobulins that bind a specific antigen. Targeted oncology monoclonal antibodies are most commonly used to target an antigen on a cancer cell, leading to downregulation of oncogene signaling, or to flag tumor cells for destruction by the immune system.8 The anti–human epidermal growth factor receptor 2 (HER2) monoclonal antibodies trastuzumab (Herceptin) and pertuzumab (Perjeta) have drastically improved outcomes for HER2-positive breast cancer, which accounts for 15% to 25% of patients with breast cancer.9 All patients with breast cancer should undergo testing for HER2 overexpression.10 Trastuzumab binds to HER2 on tumor cells, leading to internalization and down-regulation of HER2, which is a progrowth stimulator. Trastuzumab is not as effective in treating advanced gastroesophageal cancer with HER2 overexpression, offering only a 12% overall response rate.11 Cetuximab (Erbitux) is another monoclonal antibody used as targeted therapy; it binds to the epidermal growth factor receptor (EGFR), leading to downregulation of this potent growth modulator. Cetuximab and a similar anti-EGFR monoclonal antibody, panitumumab (Vectibix), are effective in treating metastatic colorectal cancer without mutations in the RAS gene because RAS mutations make tumor cells resistant to the effects of the EGFR blockade.12 Detailed testing for RAS mutations is necessary before choosing a chemotherapy regimen for metastatic colorectal cancer.
SMALL MOLECULE INHIBITORS
Small molecule inhibitors impede a vast number of targets to slow or kill tumor cells. The majority target protein kinases that are highly active progrowth signaling initiators present in all cells and are exploited by many cancers. Examples of protein kinases targeted by small molecule inhibitors include the EGFR, anaplastic lymphoma kinase, and HER2. These protein kinases are also expressed across healthy tissues, so small molecule inhibitors also have systemic effects.
Many small molecule inhibitors, such as sunitinib (Sutent), are not considered targeted therapy. This drug targets multiple, wild-type intracellular kinases and does not require testing for mutations in the tyrosine kinases that it targets (e.g., vascular endothelial growth factors).13 Alternatively, osimertinib (Tagrisso) is used only in advanced non–small cell lung cancer that contains an activating mutation in the EGFR.14
Small molecule inhibitors illustrate the ways in which the benefits of targeted therapies can range from transformational to marginal, depending on the cancer and target. For instance, when treated with osimertinib, patients with metastatic EGFR-mutated lung cancer live a median of 39 months,14 which is more than double the survival compared with similar patients treated with the first EGFR inhibitor, erlotinib (Tarceva), between 2007 and 2011.15 Similarly, patients with advanced lung cancer possessing an anaplastic lymphoma kinase fusion have a 79% response rate to the drug alectinib (Alecensa).16 At the other end of the spectrum are drugs with modest or absent survival gains. Olaparib (Lynparza) and other poly- (adenosine diphosphate-ribose) polymerase inhibitors induce double-strand DNA breaks that cannot be repaired in breast cancer gene–mutated tumors, a mechanism termed synthetic lethality. Poly- (adenosine diphosphate-ribose) polymerase inhibitors have yet to demonstrate any improvements in survival in breast cancer gene–mutated ovarian cancer.17
ANTIBODY-DRUG CONJUGATES
Antibody-drug conjugates use a monoclonal antibody bound to a cytotoxic chemotherapy molecule by a peptide linker. This allows cytotoxic therapy to be delivered directly to, and cleaved inside, a tumor cell. When tumor cells undergo apoptosis, cytotoxic chemotherapy is released, killing additional nearby tumor cells. Normal host cells in the vicinity of the tumor may also be killed; this is called the bystander effect. Additionally, amounts of the cytotoxic agent can be released prematurely into the systemic circulation. Thus, antibody-drug conjugates can still result in systemic adverse effects such as fatigue, nausea, peripheral neuropathy, and thrombocytopenia.18
IMMUNOTHERAPY
Immunotherapy is a broad term that includes monoclonal antibodies against cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), or programmed cell death ligand 1 (PD-L1), which are capable of activating the adaptive immune system against tumor cells. These immunotherapy molecules inhibit negative immune regulation and, therefore, enhance antitumor immune responses.
Immunotherapy has earned broad use. As of 2018, 43.6% of patients with cancer would be eligible for one of these drugs. Such immune checkpoint inhibitors are most commonly used in a nontargeted sense; for example, all patients with metastatic non–small cell lung cancer are likely eligible for this therapy.19 PD-L1 expression by tumor cells or tumor-infiltrating immune cells may help predict which tumors are likely to respond to immunotherapy.20 For example, the PD-L1 monoclonal antibody atezolizumab (Tecentriq), when combined with two cytotoxic chemotherapy drugs, improves survival by 10 months compared with placebo plus cytotoxic chemotherapy in patients with triple negative breast cancer and PD-L1 positive tumor–infiltrating immune cells; however, it did not improve survival in patients who had negative PD-L1 staining.21 PD-L1 is not a reliable predictor of response in many types of cancer, including metastatic non–small cell lung cancer, a disease in which immunotherapy is broadly used.22
Testing Cancer for Actionable Mutations
Specific testing of tumors for targetable alterations is critical in many advanced cancers. The initial therapy prescribed for metastatic non–small cell lung cancer will likely be an oral small molecule inhibitor if alterations in EGFR, anaplastic lymphoma kinase, receptor tyrosine kinase encoded by gene ROS1 (ROS1), or B-raf proto-oncogene (BRAF) are detected.15,23 If an elevated expression of PD-L1 is found in the tumor, immunotherapy may be used alone or in combination with cytotoxic chemotherapy.24
The initial therapy for metastatic colorectal cancer hinges on whether mutations in RAS are found (unlikely to benefit from EGFR monoclonal antibodies) and whether the tumor has mismatch repair deficiency, indicating a likely diagnosis of the hereditary Lynch syndrome and response to immunotherapy.25,26 Additionally, metastatic colorectal cancer that possesses a BRAF mutation is more aggressive, and therapy including a BRAF small molecule inhibitor should be considered.27 Other cancers for which genomic testing is critical to choosing chemotherapy include acute leukemias and metastatic bladder, breast, melanoma, ovarian, pancreatic, and prostate cancers.
Adverse Events of Targeted Therapy
Targeted oncology medications expose patients to unique toxicities, unlike the somewhat predictable adverse effects of cytotoxic chemotherapies (Table 1). One such example is ponatinib (Iclusig), which is used for the treatment of more aggressive forms of chronic myelogenous leukemia. Between 20% and 30% of patients with chronic myelogenous leukemia treated with ponatinib will experience a serious adverse event such as a heart attack, stroke, or peripheral vascular event. Taking ponatinib is linked to a 1% rate of death from these events.28 This led the FDA to briefly remove ponatinib from the market and to rerelease it with a boxed warning and reduced starting dose.29 The increasing tendency of the FDA to approve targeted cancer therapies rapidly based on early, nonrandomized evidence, as occurred with ponatinib, means that future drugs may offer unanticipated and severe adverse events.30 Patients taking ponatinib should also be taking low-dose aspirin if no contraindications exist.28
Table 2 lists possible interactions between targeted oncology medications and drugs prescribed frequently by primary care physicians. A common example is acid suppression with proton pump inhibitors when oral chemotherapeutic agents require an acidic environment for absorption.26,31–33 Solutions may include switching to histamine H2 receptor antagonists or spacing out medication dosing. Cytochrome P450, family 3A (CYP3A) inhibitors and inducers such as azoles, amiodarone, macrolide antibiotics, nondihydropyridine calcium channel blockers, antiepileptics, and antiviral medications may also lead to drug interactions.33 Often, dose reductions in the chemotherapy agent are enough to reduce the CYP3 interactions. Some oral chemotherapy drugs interact with warfarin (Coumadin), requiring closer international normalized ratio monitoring. Safe medication reconciliation requires that the oncologist, pharmacist, and primary care physician work together closely and maintain open lines of communication.
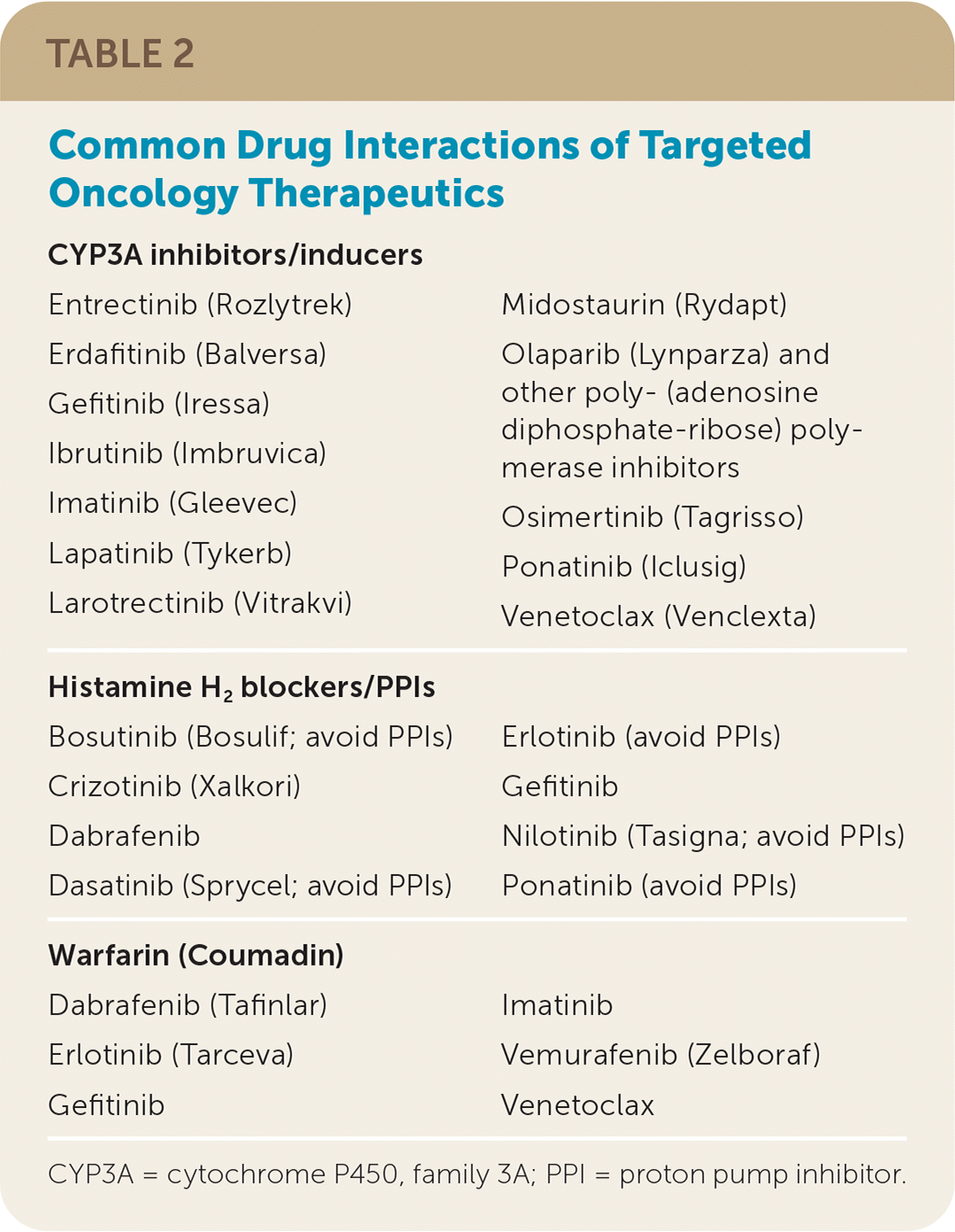
| CYP3A inhibitors/inducers | |
| Entrectinib (Rozlytrek) Erdafitinib (Balversa) Gefitinib (Iressa) Ibrutinib (Imbruvica) Imatinib (Gleevec) Lapatinib (Tykerb) Larotrectinib (Vitrakvi) Midostaurin (Rydapt) Olaparib (Lynparza) and other poly- (adenosine diphosphate-ribose) polymerase inhibitors Osimertinib (Tagrisso) Ponatinib (Iclusig) Venetoclax (Venclexta) | |
| Histamine H2 blockers/PPIs | |
| Bosutinib (Bosulif; avoid PPIs) Crizotinib (Xalkori) Dabrafenib Dasatinib (Sprycel; avoid PPIs) Erlotinib (avoid PPIs) Gefitinib Nilotinib (Tasigna; avoid PPIs) Ponatinib (avoid PPIs) | |
| Warfarin (Coumadin) | |
| Dabrafenib (Tafinlar) Erlotinib (Tarceva) Gefitinib Imatinib Vemurafenib (Zelboraf) Venetoclax |
Immunotherapy is another group of targeted therapies for which understanding the unique and unpredictable toxicities is crucial.33 Patients taking PD-1/PD-L1 or CTLA-4 monoclonal antibodies may develop autoimmune reactions of the skin (rash: 13% to 24% with pembrolizumab [Keytruda], up to 50% with ipilimumab [Yervoy]), thyroid, and other endocrine organs (hypo- or hyperthyroidism: 9% to 18% with pembrolizumab or nivolumab [Opdivo]), and more rarely gut (colitis), liver (hepatitis), lungs (pneumonitis), heart (myocarditis), brain (encephalitis), or other organs.34–36 Severe colitis requiring permanent discontinuation of immunotherapy and high-dose steroid treatment occurs in 3% to 6% of patients taking a combination of nivolumab and ipilimumab.36 Severe pneumonitis occurs in 3.2% of patients with lung cancer who are being treated with pembrolizumab.35
Immunotherapy-related adverse events may range in severity from transient to fatal and can occur at any point after receiving an immune checkpoint therapy.37 Delay in diagnosis and initiation of proper treatment—especially in cases of pneumonitis, colitis, and myocarditis—can be fatal. Evidence about which patients are likely to develop these toxicities is emerging, but patients with preexisting autoimmune conditions are at higher risk and are nearly universally excluded from immunotherapy candidacy.38 Patient and caregiver education and physician recognition of these toxicities are crucial because patients need to begin steroid therapy immediately.37
Future Directions
Targeted oncology may be moving toward a tumor-agnostic approach, in which treatments are chosen based on specific mutations in a tumor rather than the organ of origin.39 Pembrolizumab is approved for all cancers that have mismatch repair deficiency, a biomarker theorized to confer sensitivity to immune unmasking.40 The neurotrophin receptor kinase inhibitors entrectinib (Rozlytrek) and larotrectinib (Vitrakvi) are approved for all solid tumors that contain neurotrophin receptor kinase fusions.41 Such a tumor-agnostic approach has not always succeeded; vemurafenib (Zelboraf) works well for BRAF-mutated melanoma and colorectal cancer but appears ineffective in BRAF-mutated myeloma.42 It remains uncertain what effect tumor-agnostic drug approvals will have on national or global cancer outcomes.43
Financial Toxicity of Targeted Therapy
Increasingly, financial toxicity is recognized as a very real adverse effect of targeted cancer therapy. Oral cancer therapies offer the convenience of pills that can be taken at home, but they often place financial burdens on patients because of out-of-pocket costs at the pharmacy. In 2020, the average out-of-pocket cost to a patient for a course of oral cancer therapy was estimated at $5,663.44 According to one large analysis, 20% of patients with cancer take less medication than prescribed, 19% partially fill oral cancer therapy prescriptions, and 24% avoid filling a prescription at all.45 Many patients in this study reported spending less money on food, leisure, and clothing.45 Approximately 2% of patients will declare bankruptcy during their treatment; those with advanced disease are more likely to declare bankruptcy.46 Bankruptcy during cancer treatment increases the risk of death.46 Beyond financial toxicity to the individual patient, the effects of targeted oncology on escalating health care costs are significant. Immunotherapy is not cost-effective in some studies,47 and testing of advanced cancers using next-generation sequencing assays may substantially increase the cost of care without meaningfully affecting survival.48 Primary care physicians can help patients by assessing for financial toxicity and by offering suggestions such as drug company assistance, charity care, and social work resources to make cancer treatment more affordable.
Data Sources: A PubMed search was completed in Clinical Queries using the key terms precision oncology, metastatic non–small cell lung cancer, BRCA, breast cancer, ovarian cancer, HER2-positive breast cancers; locally advanced and metastatic melanoma, metastatic colorectal cancer, chronic myeloid leukemia, NTRK fusion solid tumors, microsatellite unstable solid tumors, tumor agnostic, oral cancer drug interactions, immunotherapy toxicity, and financial toxicity. Also searched were the National Comprehensive Cancer Network guidelines, UpToDate, Essential Evidence Plus, and the U.S. Food and Drug Administration website. Search dates: March 7 and October 13, 2020.
