
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2021;103(9):553-558
Patient information: See related handout on urethritis, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Urethritis refers to inflammation of the urethra and is classified as gonococcal (caused by Neisseria gonorrhoeae) or nongonococcal in origin (most commonly caused by Chlamydia trachomatis, Mycoplasma genitalium, or Trichomonas vaginalis). The most common signs and symptoms include dysuria, mucopurulent urethral discharge, urethral discomfort, and erythema. Diagnostic criteria include typical signs, symptoms, or history of exposure in addition to mucopurulent discharge, Gram stain of urethral secretions showing at least two white blood cells per oil immersion field, first-void urinalysis showing at least 10 white blood cells per high-power field, or a positive leukocyte esterase result with first-void urine. First-line empiric treatment consists of ceftriaxone and doxycycline; however, the antibiotic regimen may be targeted to the isolated organism. Repeat testing is not recommended less than three weeks after treatment because false-positive results are possible during this time. Patients treated for a sexually transmitted infection should have repeat screening in three months, with shared decision-making about future screening intervals. Patients treated for urethritis should abstain from sex for seven days after the start of treatment, until their partners have been adequately treated, and until their symptoms have fully resolved.
Urethritis refers to inflammation of the urethra. It is classified as gonococcal (caused by Neisseria gonorrhoeae) or nongonococcal in origin. Nongonococcal urethritis can be caused by several other organisms; Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis are the most common. The differential diagnosis of urethritis is summarized in Table 1.
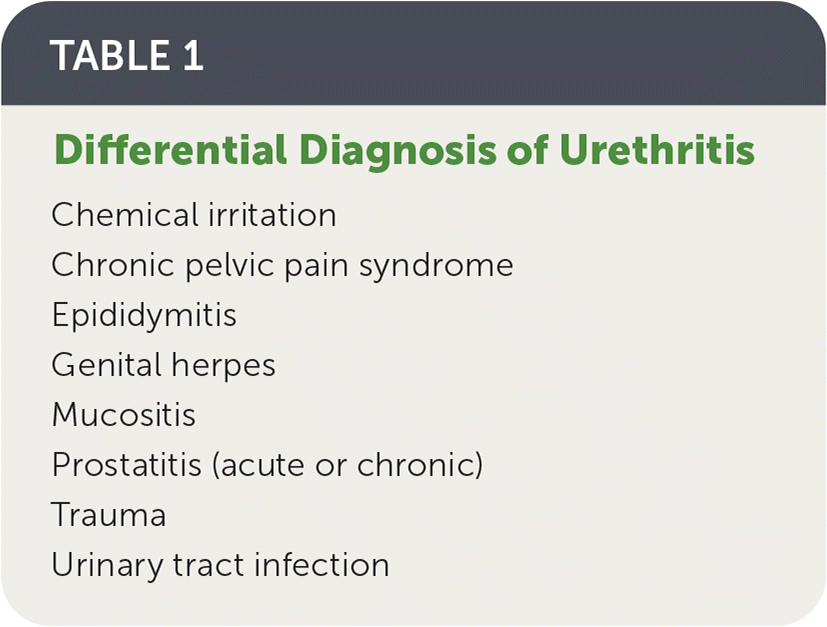
| Chemical irritation |
| Chronic pelvic pain syndrome |
| Epididymitis |
| Genital herpes |
| Mucositis |
| Prostatitis (acute or chronic) |
| Trauma |
| Urinary tract infection |
Epidemiology
In 2018, the incidence of chlamydial urethritis was 381 cases per 100,000 U.S. men, making it the most common reportable condition and the most common type of urethritis in men. The number of reported cases of chlamydia increased 36% in U.S. men and women from 2008 to 2018.1
In 2018, the incidence of gonococcal urethritis was 213 cases per 100,000 U.S. men.1,2
Table 2 lists common bacterial isolates in patients with nongonococcal urethritis.3 Approximately one-half of nongonococcal cases have no clear etiology.
M. genitalium causes 15% to 20% of nongonococcal urethritis cases and higher rates of recurrent urethritis.4 The prevalence of M. genitalium isolates is 1.3% in the general population and 3.2% in men who have sex with men (MSM).5 M. genitalium is more common in men who are younger, who smoke, and who have multiple sex partners.6
T. vaginalis is a less common cause of urethritis. It was present in urine samples from 0.5% of men in the 2013–2016 cohort of the National Health and Nutrition Examination Survey,7 and its prevalence is as high as 6.6% to 11% in metropolitan areas with high prevalence of sexually transmitted infections (STIs).8,9 T. vaginalis infection is more common among people who are older or incarcerated; men who have sex with women; people with multiple sex partners; and when the local prevalence is high (more than 2% in symptomatic women).3,4,9,10
Ureaplasma urealyticum infection is associated with nongonococcal urethritis.11
Herpes simplex virus, adenovirus, and Haemophilus influenzae are rare causes of urethritis.
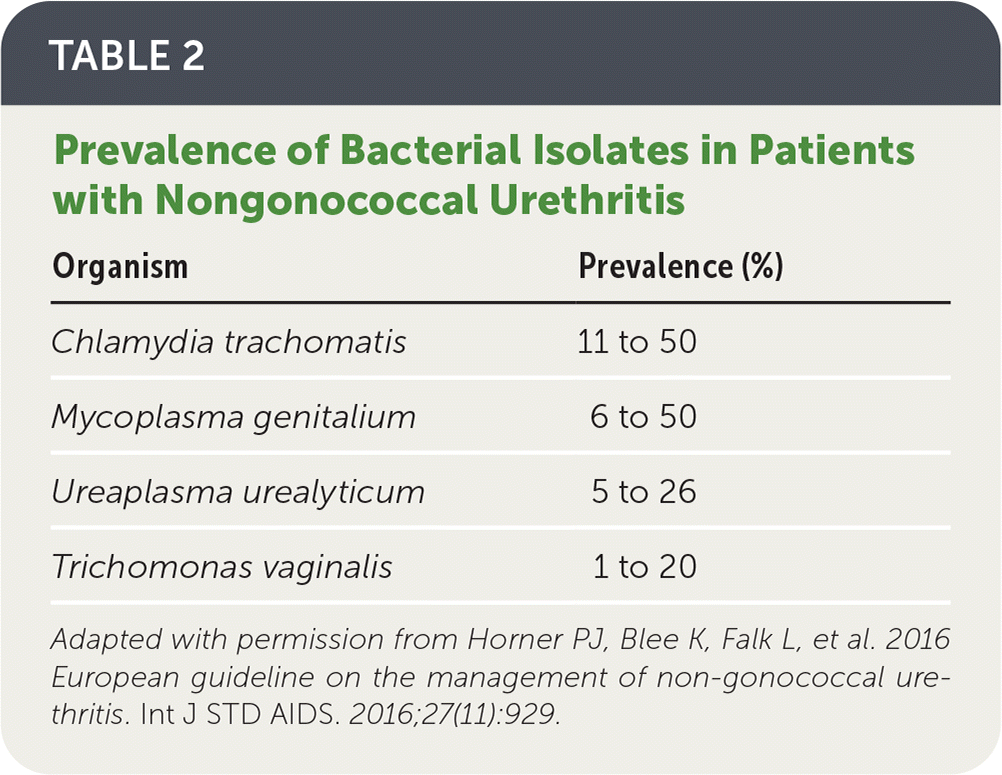
| Organism | Prevalence (%) |
|---|---|
| Chlamydia trachomatis | 11 to 50 |
| Mycoplasma genitalium | 6 to 50 |
| Ureaplasma urealyticum | 5 to 26 |
| Trichomonas vaginalis | 1 to 20 |
Clinical Features
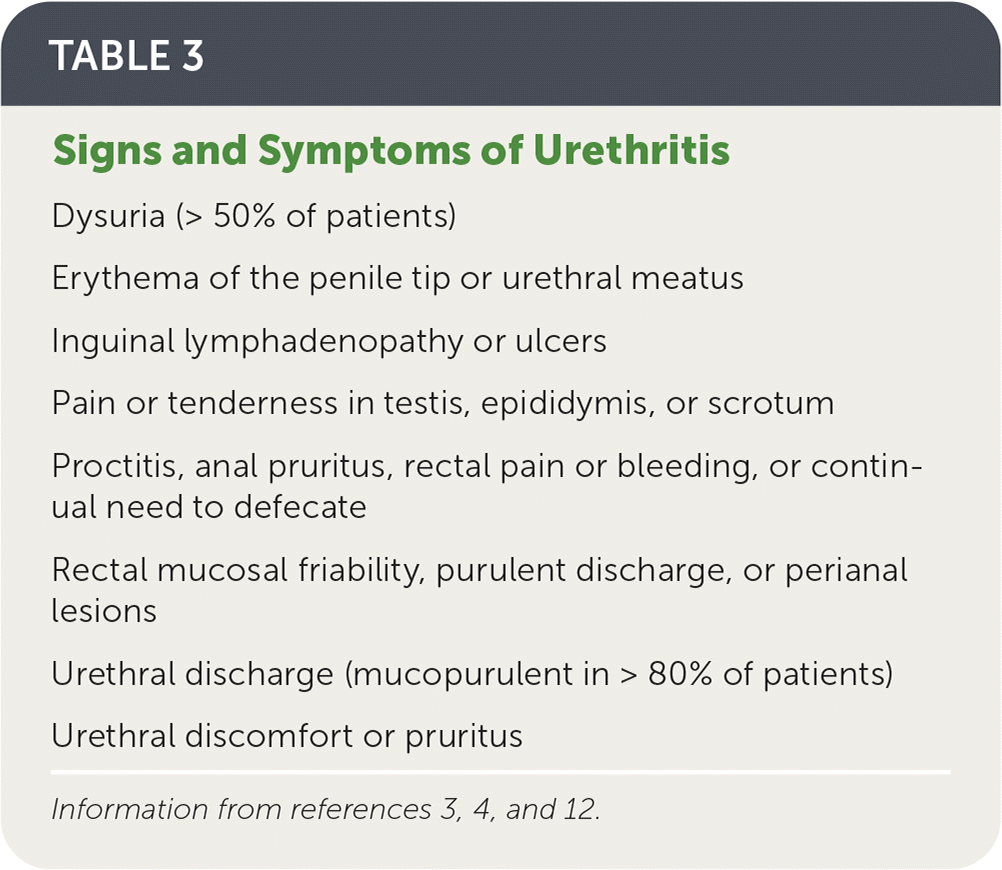
| Dysuria (> 50% of patients) |
| Erythema of the penile tip or urethral meatus |
| Inguinal lymphadenopathy or ulcers |
| Pain or tenderness in testis, epididymis, or scrotum |
| Proctitis, anal pruritus, rectal pain or bleeding, or continual need to defecate |
| Rectal mucosal friability, purulent discharge, or perianal lesions |
| Urethral discharge (mucopurulent in > 80% of patients) |
| Urethral discomfort or pruritus |
Diagnosis
In addition to examination of the genital area in people with urethritis symptoms, the Centers for Disease Control and Prevention (CDC) recommends examination of the skin, pharynx, lymph nodes, anogenital area, and neurologic system when evaluating for STIs.13
Urethritis should be suspected based on the presence of typical signs and symptoms, such as dysuria, urethral discharge, or urethral erythema.
The diagnosis can be confirmed using the criteria listed in Table 4.4
First-void urine samples are preferred when testing for N. gonorrhoeae or C. trachomatis infection using a nucleic acid amplification test (NAAT).4,14 First-catch urine (i.e., the initial 10 to 20 mL of the urinary stream, ideally collected at least 20 to 60 minutes after the last micturition) is a reasonable alternative with similar sensitivity.15–17 In both methods, the urethral meatus should not be cleaned before the sample is collected.
Urine culture with antimicrobial sensitivity testing should be reserved for cases of gonorrhea with suspected antimicrobial resistance.
Gonococcal urethritis is indicated by the presence of polymorphonuclear cells and gram-negative diplococci on Gram stain of urethral secretions.
Testing for T. vaginalis infection by urethral swab culture or NAAT should be considered for people with recurrent urethritis or in areas or populations with a high prevalence. NAAT has higher sensitivity and specificity than culture.
The U.S. Food and Drug Administration approved a test for M. genitalium infection in January 2019,18 but no randomized trials have evaluated the cost effectiveness of routinely testing patients with urethritis. The CDC recommends that M. genitalium infection be considered in cases of persistent or recurrent urethritis, even if testing is not available locally.4
Testing for U. urealyticum infection in patients with urethritis is not routinely recommended because a positive test result does not confirm causality.3,11
Testing for syphilis, HIV infection, and hepatitis B should be considered for patients with urethritis and STI risk factors (e.g., multiple sex partners, suspected exposure, MSM).4
In sexually active MSM with chlamydia or gonorrhea diagnosed by NAAT, extragenital (oral and/or anal) test results are positive in more than 70% of those with normal urine samples.2,19
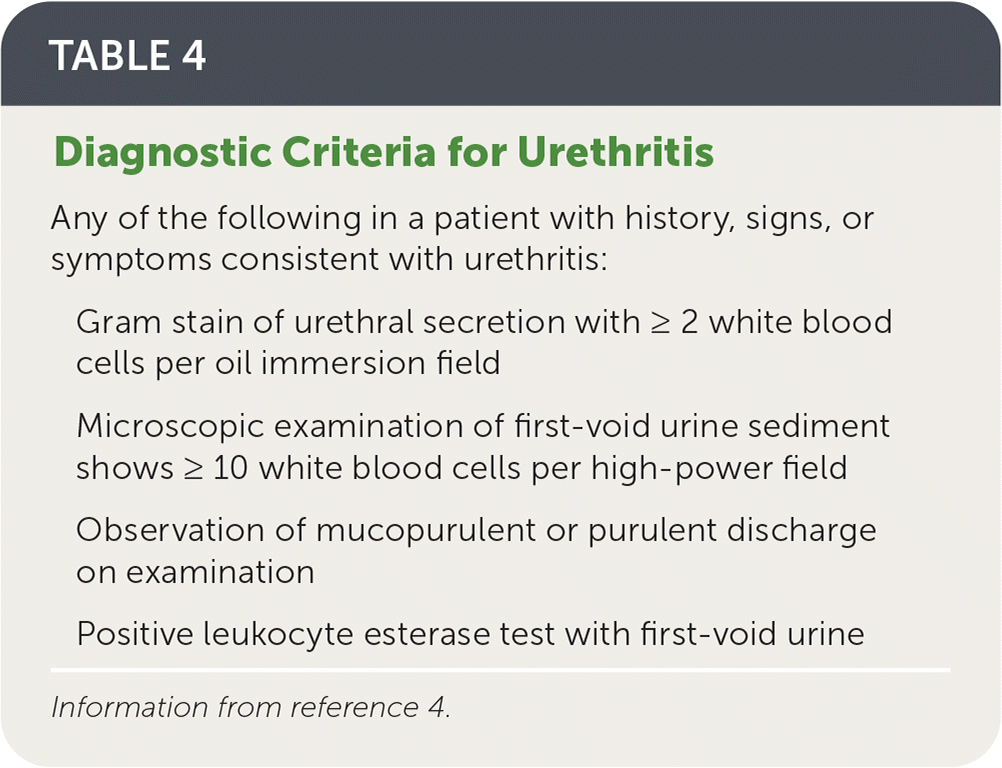
| Any of the following in a patient with history, signs, or symptoms consistent with urethritis: |
| Gram stain of urethral secretion with ≥ 2 white blood cells per oil immersion field |
| Microscopic examination of first-void urine sediment shows ≥ 10 white blood cells per high-power field |
| Observation of mucopurulent or purulent discharge on examination |
| Positive leukocyte esterase test with first-void urine |
Treatment
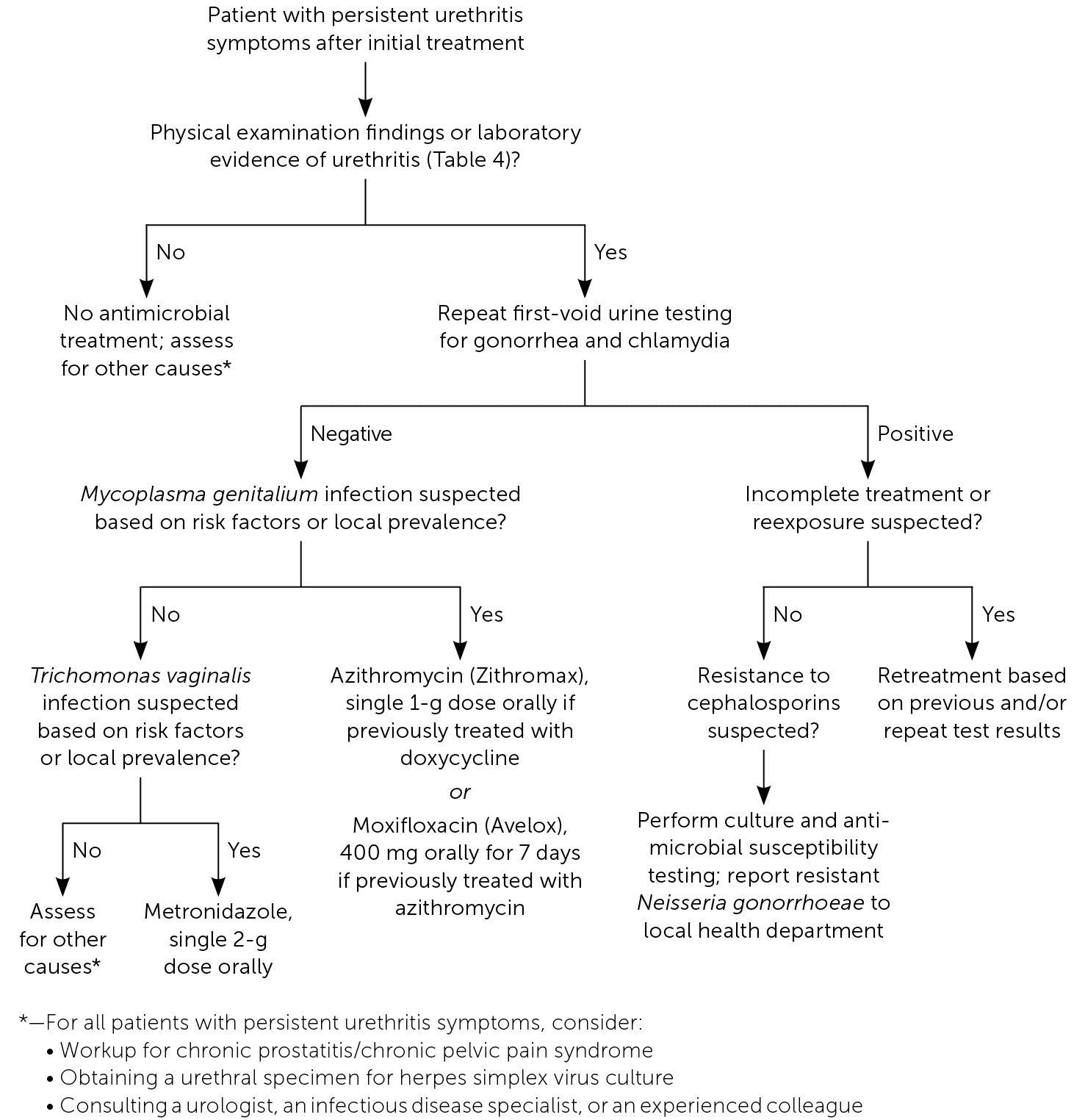
Treatment recommendations for common causes of urethritis are summarized in Table 5.4,20 A suggested approach to patients with persistent symptoms after initial treatment is presented in Figure 1.21 [corrected]
The preferred agents for empiric treatment of urethritis are intramuscular ceftriaxone, one 500-mg dose, plus oral doxycycline, 100 mg twice per day for seven days.4,20 For patients with gonorrhea, appropriate therapy is recommended to prevent resistance, which is emerging internationally.4
Intramuscular gentamicin, 240 mg, is inferior to intramuscular ceftriaxone, 500 mg, for the treatment of gonorrhea when either agent is used in combination with oral azithromycin (Zithromax), 1 g.22
Alternative treatment regimens for oral gonorrhea are inferior to first-line treatment options. If an alternative regimen is prescribed, a test of cure should be performed 14 days later.4
A 2019 Cochrane review found that a seven-day course of doxycycline therapy in men with chlamydia resulted in fewer treatment failures (defined as the need for repeat testing one to four weeks after treatment, indicating persistent chlamydia) than a single dose of azithromycin, but doxycycline had a higher rate of adverse effects.23 However, there were no differences in rates of persistent urethritis symptoms after treatment.
Reinfection with N. gonorrhoeae is more common than treatment failure. However, if treatment failure is suspected, a culture and antimicrobial resistance testing can be useful for guiding further treatment and reporting to the local health department.
Single-dose treatment regimens have higher rates of adherence. Direct observation of treatment initiation can increase adherence to multidose regimens.
Expedited partner therapy (prescribing treatment for sex partners of patients with newly diagnosed chlamydia or gonorrhea without examining the partner) is legal in most states.24 Compared with independently telling patients to notify their partners to seek treatment, this strategy is more effective for reducing rates of recurrent urethritis in patients with gonorrhea, chlamydia, or nongonococcal urethritis.25,26
NAAT can have false-positive results when performed less than three weeks after a patient has completed treatment for gonorrhea or chlamydia; therefore, a test of cure is not advised during this time.4 Repeat testing for M. genitalium can be considered three to five weeks after completing treatment for a confirmed infection because of this organism's potential antimicrobial resistance.3
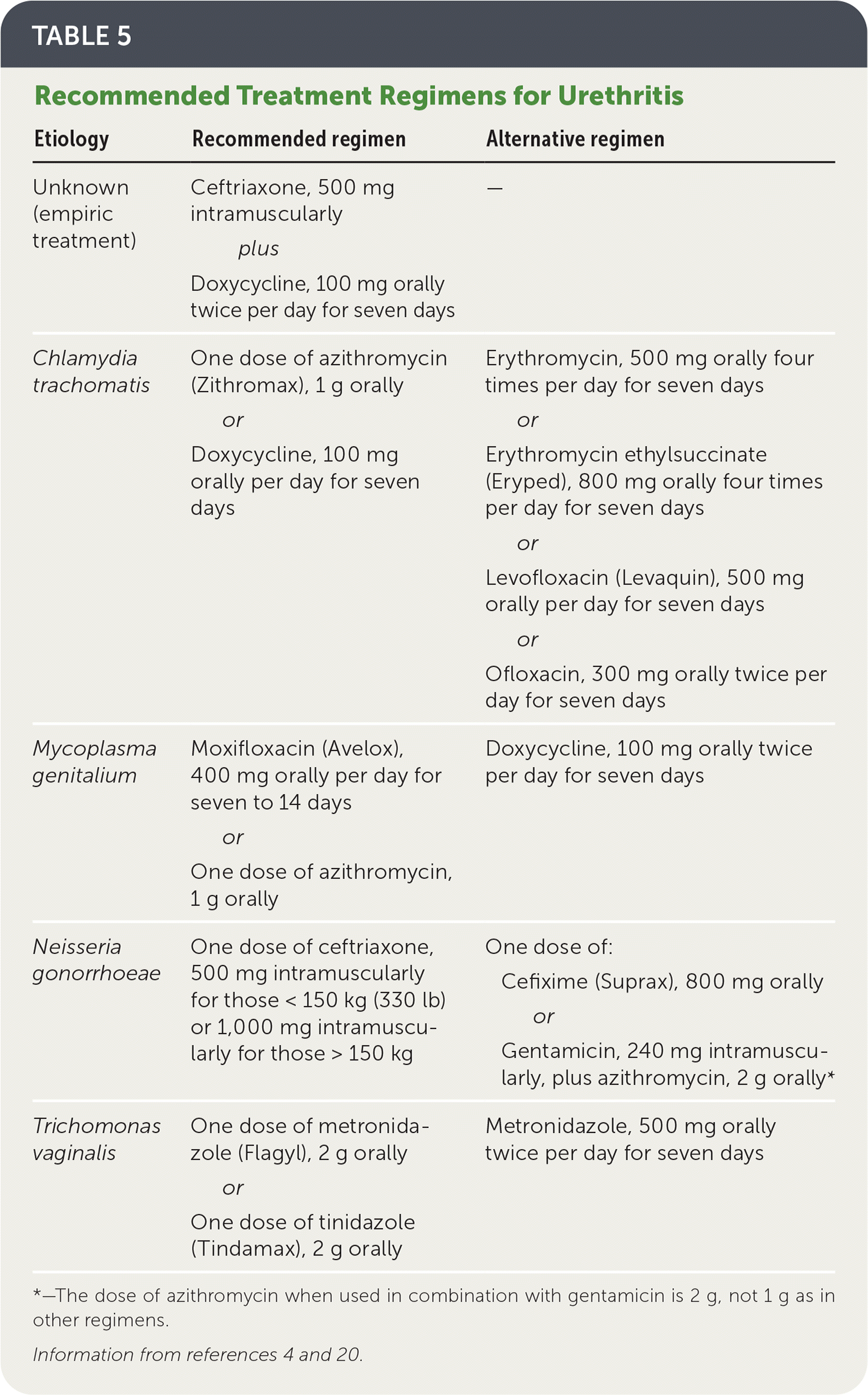
| Etiology | Recommended regimen | Alternative regimen |
|---|---|---|
| Unknown (empiric treatment) | Ceftriaxone, 500 mg intramuscularly plus Doxycycline, 100 mg orally twice per day for seven days | — |
| Chlamydia trachomatis | One dose of azithromycin (Zithromax), 1 g orally or Doxycycline, 100 mg orally per day for seven days | Erythromycin, 500 mg orally four times per day for seven days or Erythromycin ethylsuccinate (Eryped), 800 mg orally four times per day for seven days or Levofloxacin (Levaquin), 500 mg orally per day for seven days or Ofloxacin, 300 mg orally twice per day for seven days |
| Mycoplasma genitalium | Moxifloxacin (Avelox), 400 mg orally per day for seven to 14 days or One dose of azithromycin, 1 g orally | Doxycycline, 100 mg orally twice per day for seven days |
| Neisseria gonorrhoeae | One dose of ceftriaxone, 500 mg intramuscularly for those < 150 kg (330 lb) or 1,000 mg intramuscularly for those > 150 kg | One dose of: Cefixime (Suprax), 800 mg orally or Gentamicin, 240 mg intramuscularly, plus azithromycin, 2 g orally* |
| Trichomonas vaginalis | One dose of metronidazole (Flagyl), 2 g orally or One dose of tinidazole (Tindamax), 2 g orally | Metronidazole, 500 mg orally twice per day for seven days |
Screening
The CDC recommends repeat screening three months after treatment for an STI,4 with shared decision-making about future screening intervals based on individual sex behaviors, HIV status, number of sex partners, and patient preference.
The CDC recommends screening for chlamydia in sexually active younger people in high-prevalence areas or populations,4 whereas the U.S. Preventive Services Task Force (USPSTF) found that the current evidence is insufficient to assess the balance of benefits and harms.27
Annual screening for chlamydia and gonorrhea is recommended for sexually active MSM based on their exposure history, with more frequent screening in populations at highest risk.4
Prevention
The CDC recommends routinely obtaining patients' sexual histories and, if indicated, providing risk-reduction strategies, including prevention counseling.4
The USPSTF recommends intensive behavioral counseling for all sexually active adolescents and for adults at increased risk of STIs.28
Patients treated for an STI should abstain from sex for seven days after treatment begins, until their partners have been adequately treated, and until symptoms have fully resolved.4,29
Vaccination for other vaccine-preventable STIs (e.g., human papillomavirus, hepatitis A and B infections) should be considered for patients with urethritis and ongoing risk factors.
Consistent condom use (male or female) can reduce the risk of future STIs. Abstinence can prevent future STIs. In sub-Saharan Africa, male circumcision has been shown to reduce the risk of genital ulcer disease and human papillomavirus infection in men who have sex with women, but not transmission of N. gonorrhoeae or C. trachomatis infection.30
Preexposure and postexposure prophylaxis for HIV infection should be discussed with patients with diagnosed STIs who have ongoing risk factors.31
Postexposure prophylaxis with a single 200-mg dose of doxycycline reduces rates of chlamydia and syphilis, but not gonorrhea, in MSM who do not use condoms.32
Data Sources: A PubMed search was completed in Clinical Queries using the key terms urethritis, chlamydia, Neisseria gonorrhoeae, Mycoplasma, and Trichomonas. Essential Evidence Plus and the Cochrane database were also searched, as were reference lists in retrieved articles. Search dates: October 2019 to June 2020.
