
Am Fam Physician. 2021;104(2):152-159
Author disclosure: No relevant financial affiliations.
With more than 200 types identified, human papillomavirus (HPV) commonly causes infections of the skin and mucosa. HPV infection is the most common sexually transmitted infection in the United States. Although most HPV infections are transient and subclinical, some lead to clinical manifestations ranging from benign papillomas or warts to intraepithelial lesions. In some patients, persistent infection with high-risk mucosal types, especially HPV-16 and HPV-18, causes anal, cervical, oropharyngeal, penile, vaginal, and vulvar cancers. Most HPV-related cancers are believed to be caused by sexual spread of the virus. A history of multiple sex partners; initiation of sexual activity at an early age; not using barrier protection; other sexually transmitted infections, including HIV; an immunocompromised state; alcohol use; and smoking have been identified as risk factors for persistent HPV infections. Screening for HPV infection is effective in identifying precancerous lesions and allows for interventions that can prevent the development of cancer. Use of condoms and dental dams may decrease spread of the virus. Vaccination is the primary method of prevention. The nonavalent HPV vaccine is effective in preventing the development of high-grade precancerous cervical lesions in noninfected patients. Vaccination is ideally administered at 11 or 12 years of age, irrespective of the patient's sex. In general, a two-dose series is recommended if administered before 15 years of age; however, individuals who are immunocompromised require three doses.
There are more than 200 types of human papillomavirus (HPV), a DNA virus that infects cutaneous and mucosal epithelial cells. HPV is spread by direct skin-to-skin contact and has tropisms for cutaneous or mucosal epithelial cells.1 A small subset of HPV types can cause cutaneous warts.2 The approximately 40 types that infect mucosal surfaces are typically spread through sexual contact, including vaginal, anal, or oral sex, and can be divided into low-risk and high-risk types based on their associated cancer risk. Low-risk types cause warts, whereas the 15 high-risk types cause cervical intraepithelial neoplasia (CIN) and squamous cell carcinomas of the anogenital tract and oropharyngeal mucosa.3,4 Vertical or horizontal spread of HPV can occur during the perinatal period and is associated with oral infections and respiratory papillomatosis.5,6 Concomitant cervical and anal infections have been demonstrated in women without a history of anal intercourse and may be a result of autoinoculation.7
WHAT'S NEW ON THIS TOPIC
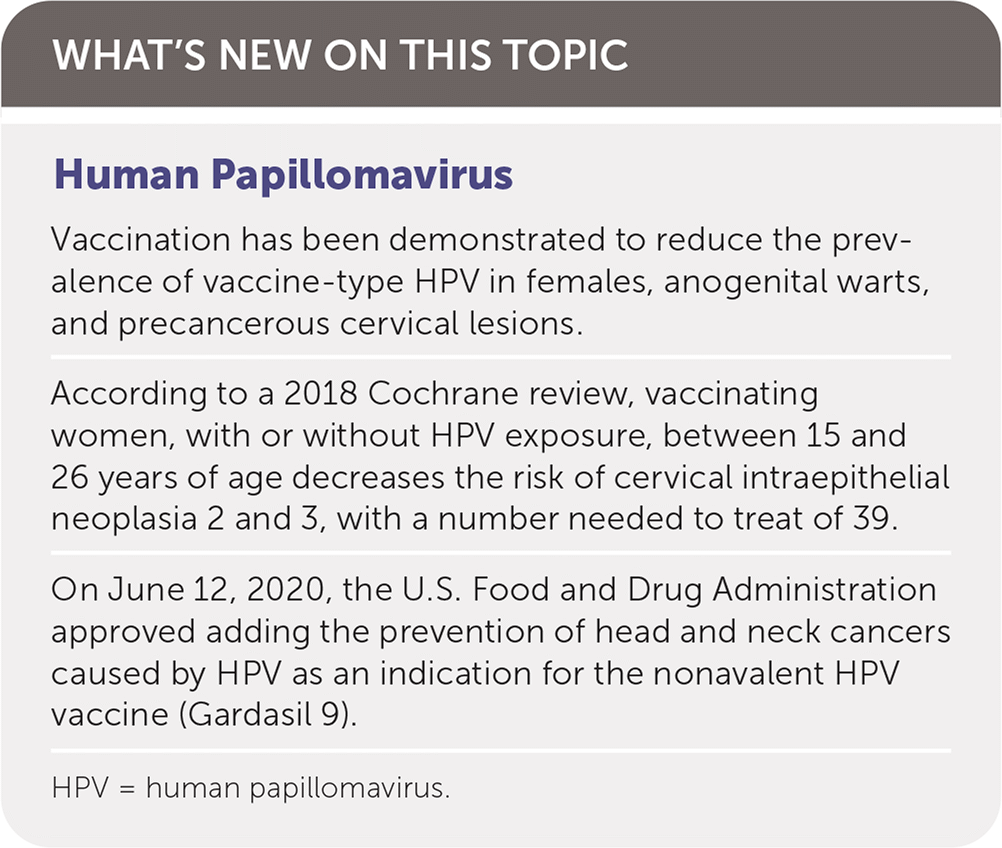
Human Papillomavirus
Vaccination has been demonstrated to reduce the prevalence of vaccine-type HPV in females, anogenital warts, and precancerous cervical lesions.
According to a 2018 Cochrane review, vaccinating women, with or without HPV exposure, between 15 and 26 years of age decreases the risk of cervical intraepithelial neoplasia 2 and 3, with a number needed to treat of 39.
On June 12, 2020, the U.S. Food and Drug Administration approved adding the prevention of head and neck cancers caused by HPV as an indication for the nonavalent HPV vaccine (Gardasil 9).
HPV = human papillomavirus.
| Recommendation | Sponsoring organization |
|---|---|
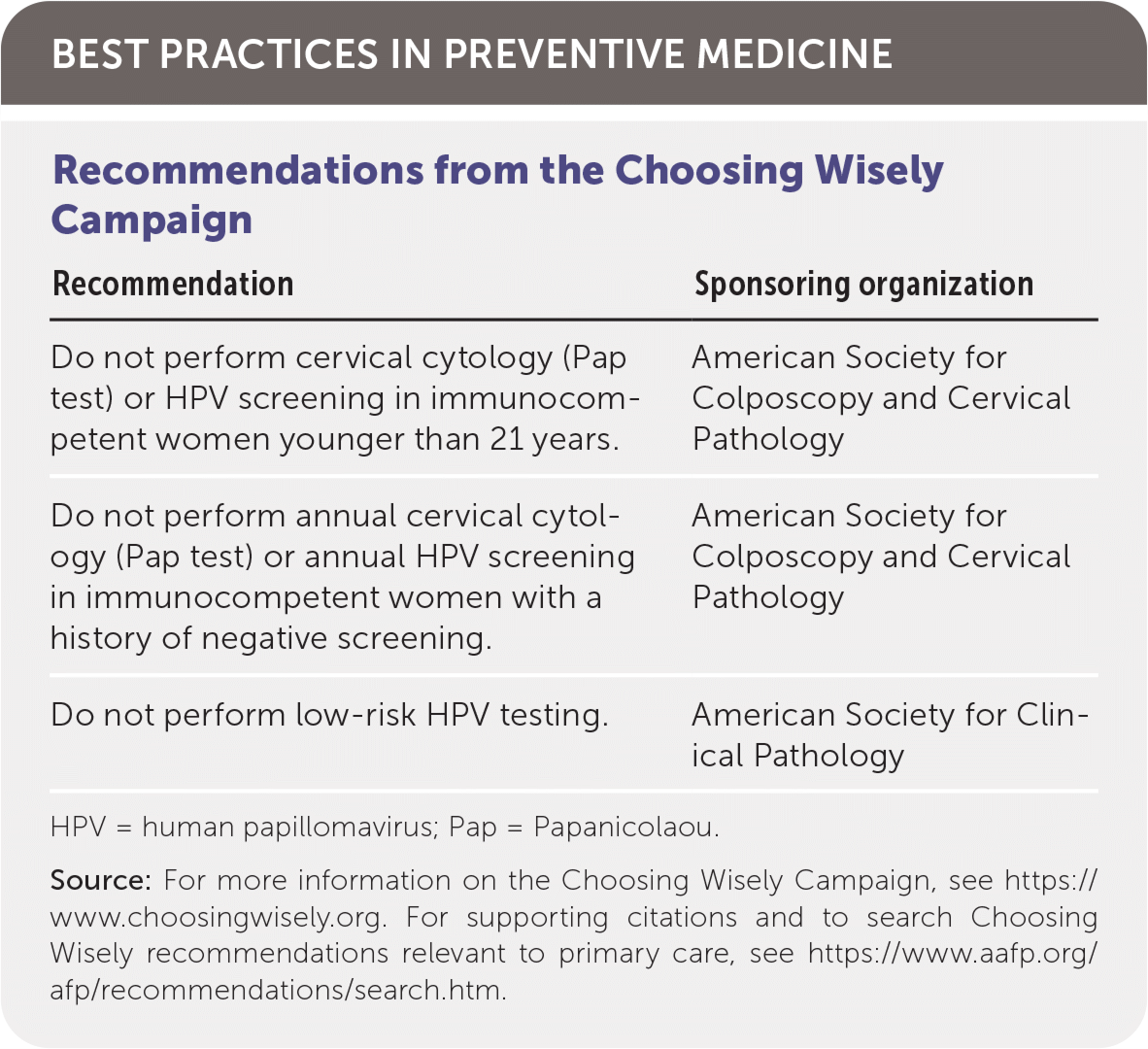
| Do not perform cervical cytology (Pap test) or HPV screening in immunocompetent women younger than 21 years. | American Society for Colposcopy and Cervical Pathology |
| Do not perform annual cervical cytology (Pap test) or annual HPV screening in immunocompetent women with a history of negative screening. | American Society for Colposcopy and Cervical Pathology |
| Do not perform low-risk HPV testing. | American Society for Clinical Pathology |
Epidemiology and Prevalence
The Centers for Disease Control and Prevention reports that 79 million Americans are infected with HPV and an additional 14 million are newly infected each year.3 Data from early HPV vaccine trials suggest that the lifetime prevalence of the infection is 85% in women and 91% in men who have had at least one sex partner.8
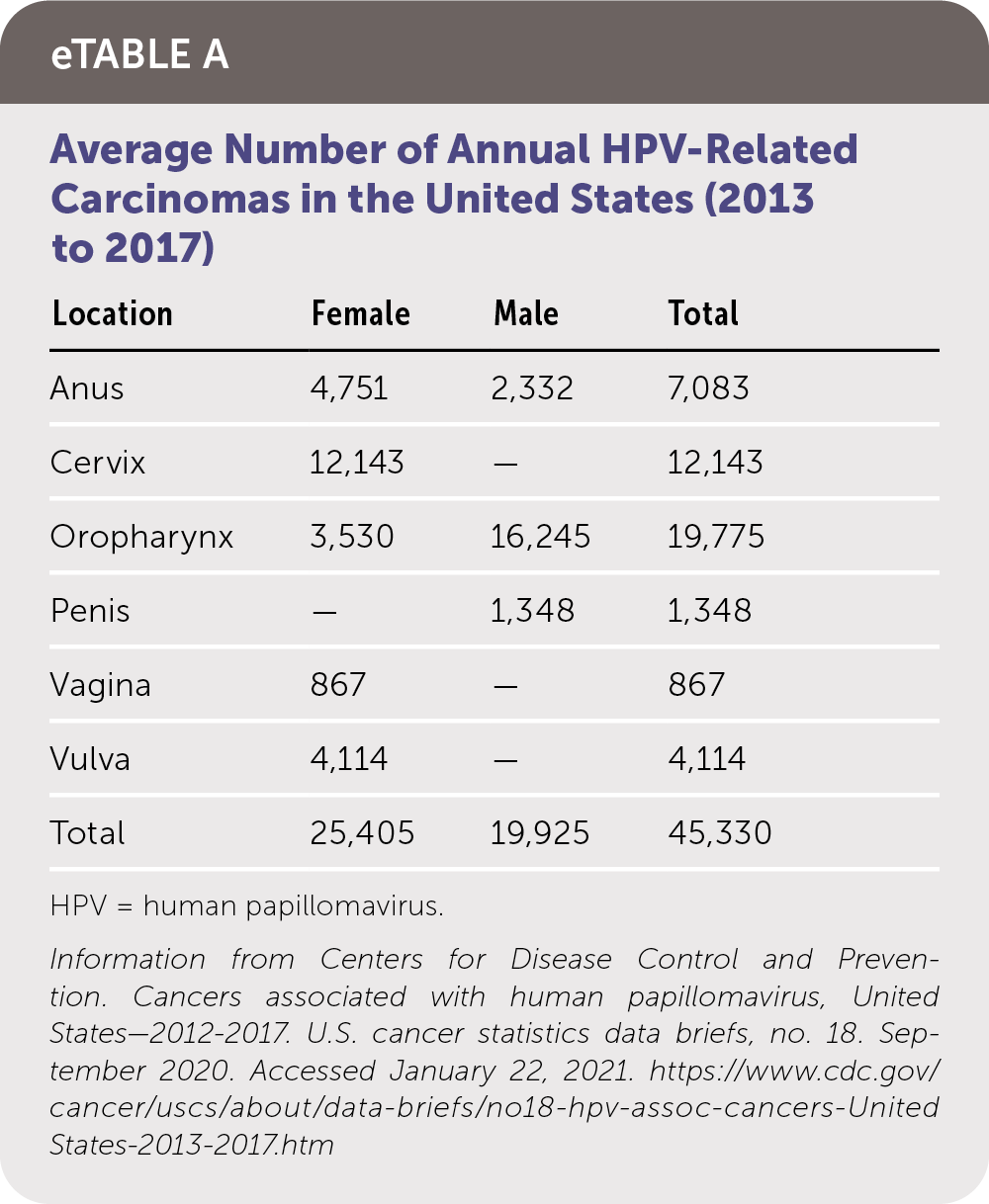
The prevalence of cutaneous warts is highest in school-aged children (up to 30%), then declines with advancing age.2 HPV infection is the most common sexually transmitted infection in the United States. Genital warts occur in 1% of sexually active adults.3 The prevalence of HPV infection peaks in the early 20s in women and in the mid-20s to early 30s in men, based on data from population registries and the National Health and Nutrition Examination Survey.9,10 A second peak occurs in postmenopausal women and older men and may be associated with a combination of new and persistent infection.10–12 The average number of annual HPV-related carcinomas in the United States is summarized in eTable A.
| Location | Female | Male | Total |
|---|---|---|---|
| Anus | 4,751 | 2,332 | 7,083 |
| Cervix | 12,143 | — | 12,143 |
| Oropharynx | 3,530 | 16,245 | 19,775 |
| Penis | — | 1,348 | 1,348 |
| Vagina | 867 | — | 867 |
| Vulva | 4,114 | — | 4,114 |
| Total | 25,405 | 19,925 | 45,330 |
Risk Factors
INFECTION
PERSISTENCE
Persistent oral and genital HPV infections are associated with alcohol use and smoking.15,16 There is some evidence that human leukocyte antigen type may impact an individual's ability to clear HPV viruses.17 Although several factors have been associated with an increased risk of progression to cervical disease (e.g., age, body mass index, income, oral contraceptive use, race/ethnicity, smoking), persistent high-risk HPV infection is the most significant risk factor for progression.18,19
Pathogenesis and Subtypes
Infection with a low-risk HPV type does not preclude infection with a concomitant high-risk type. One study demonstrated that 31% of genital warts contain both low- and high-risk types of HPV.20
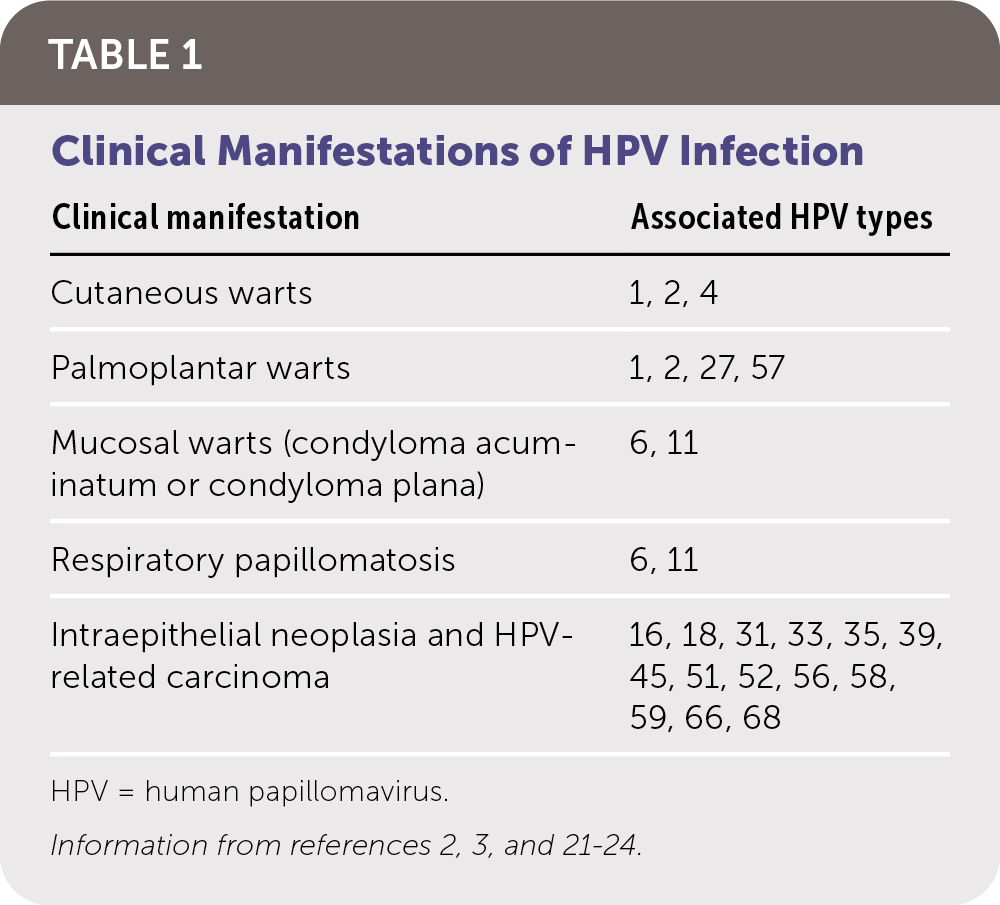
HPV infection can be latent and subclinical or have a presentation ranging from benign cutaneous and mucosal lesions to life-threatening clinical carcinomas (Table 12,3,21–24). The risk of CIN and HPV-related carcinomas is the primary clinical concern. Table 2 summarizes HPV-related carcinomas and associated HPV types.4,22
| Clinical manifestation | Associated HPV types |
|---|---|
| Cutaneous warts | 1, 2, 4 |
| Palmoplantar warts | 1, 2, 27, 57 |
| Mucosal warts (condyloma acuminatum or condyloma plana) | 6, 11 |
| Respiratory papillomatosis | 6, 11 |
| Intraepithelial neoplasia and HPV-related carcinoma | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 |
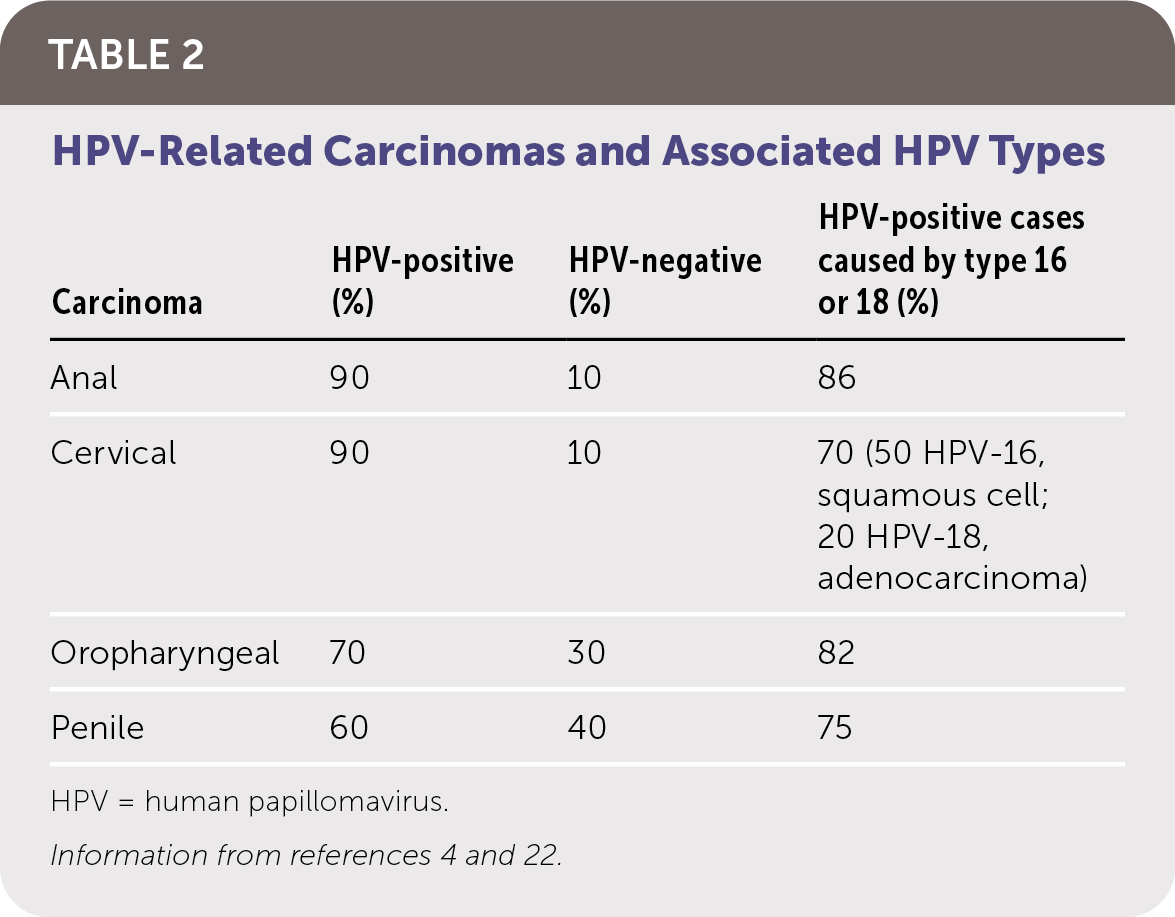
| Carcinoma | HPV-positive (%) | HPV-negative (%) | HPV-positive cases caused by type 16 or 18 (%) |
|---|---|---|---|
| Anal | 90 | 10 | 86 |
| Cervical | 90 | 10 | 70 (50 HPV-16, squamous cell; 20 HPV-18, adenocarcinoma) |
| Oropharyngeal | 70 | 30 | 82 |
| Penile | 60 | 40 | 75 |
Natural History and Timeline
Most of what is known about the natural history of HPV infections is a result of the extensive study of genital tract disease in women. Data suggest that oral infections have a similar natural history.5 In 90% of individuals with low- or high-risk HPV infection, the immune system clears the virus within two years.25,26 It is unclear whether this represents ongoing infection, which is permanently suppressed, or immune-mediated elimination of the infection.27
Up to 30% of genital warts spontaneously regress within four months, and 50% of cutaneous warts in children spontaneously resolve within one year.28,29 The decision to treat warts depends on the size, number, and persistence of the warts and on patient preference.29 Treatment is up to 80% effective and can result in quicker resolution.30,31
The cervical cytology of HPV infection progresses from atypical squamous cells of undetermined significance (ASCUS) to carcinoma. Because there is a long latent period (10 to 20 years) between cervical HPV infection and diagnosis, the incidence of carcinoma peaks at 40 years of age.11,32 This highlights the importance of screening and testing for HPV-related cervical changes between 30 and 40 years of age.11
Although ASCUS is the most benign pathologic categorization on a Papanicolaou (Pap) smear, approximately 50% of ASCUS findings are associated with high-risk HPV infections.33 CIN (or cervical dysplasia), which typically occurs at the squamocolumnar junction, indicates an active HPV infection and is considered precancerous. CIN is graded histologically from 1 to 3 based on the extent of epithelial involvement, with 3 representing the most severe dysplasia. CIN 1 and 2 often regress or resolve without treatment.34 However, infections that last longer than one or two years are more likely to progress to a higher-grade CIN or carcinoma.35 Between 12% and 30% of CIN 3 lesions will progress to invasive cervical carcinoma.36 Concurrent infection with multiple high-risk types of HPV can have a synergistic effect on the progression to cervical cancer.37
HPV-positive oropharyngeal carcinomas present at a younger age than HPV-negative cancers. Although HPV-positive cancers have a less specific presentation, they are more responsive to treatment and have a higher survival rate than HPV-negative cancers.38
Screening
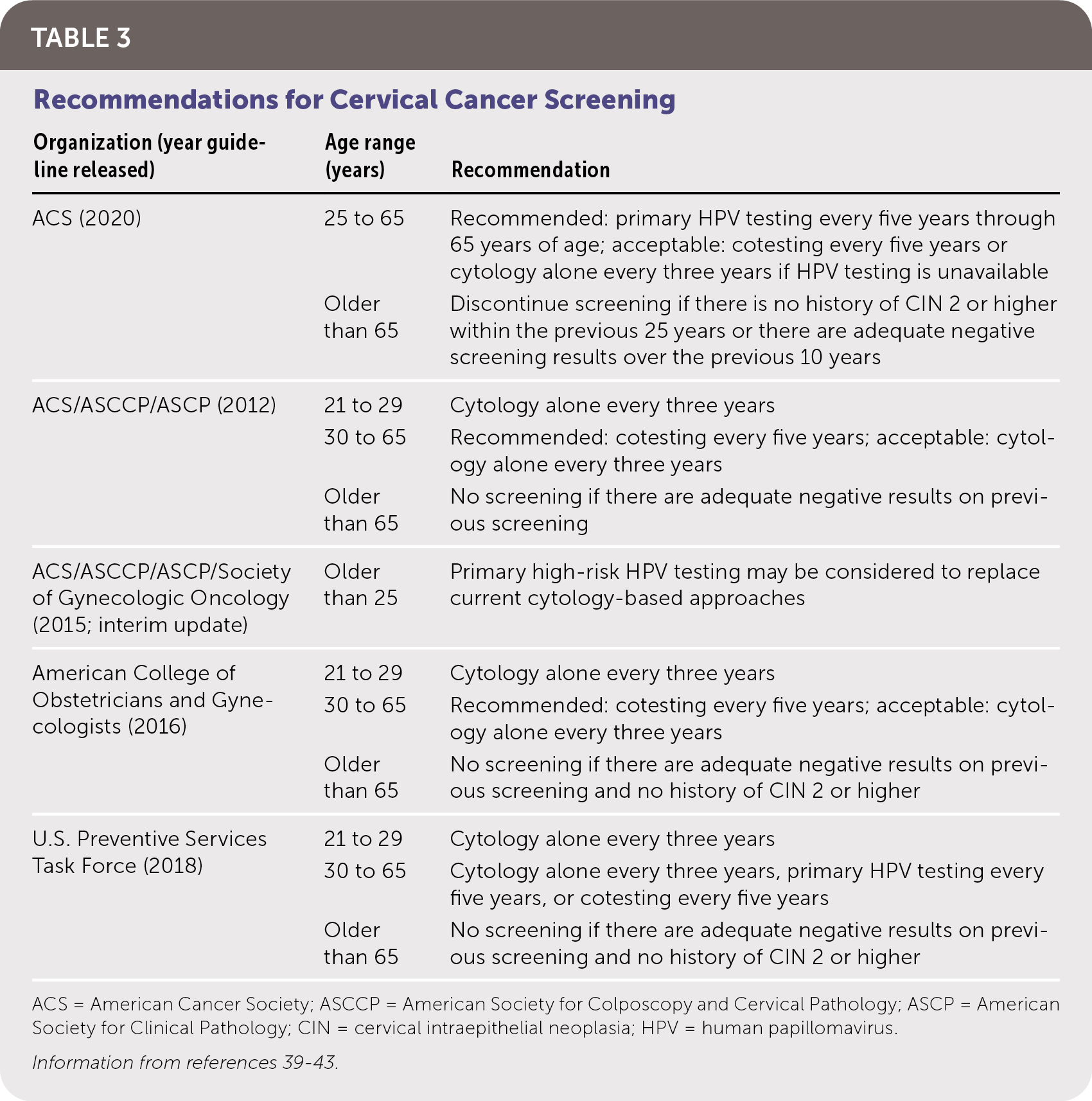
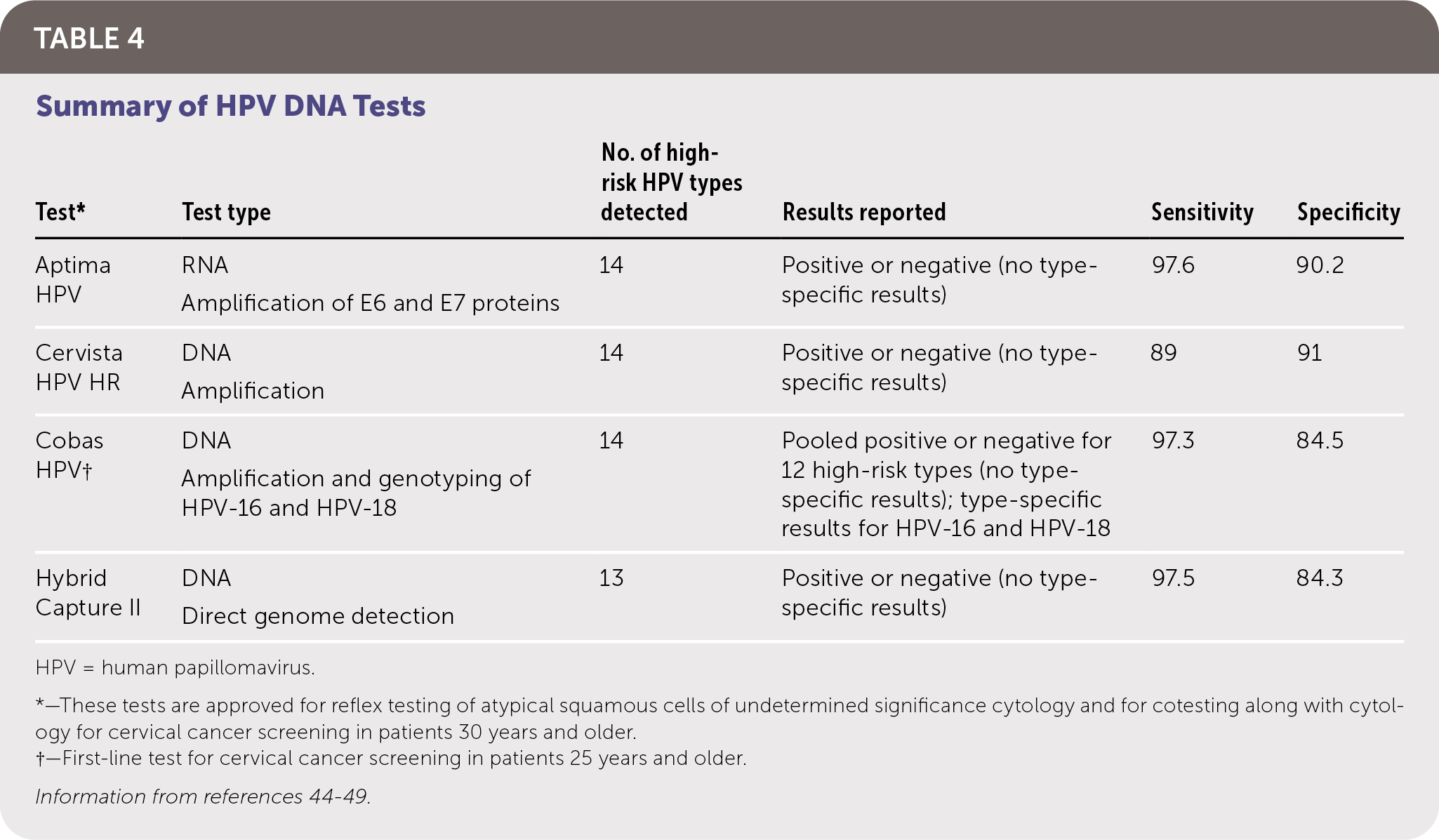
The goal of screening for HPV is to identify precancerous lesions, allowing for treatment to prevent progression to carcinoma. Options for screening include cytology-based testing (Pap smear), high-risk HPV testing, and cotesting (simultaneous cytology and high-risk HPV testing). Recommendations for cervical cancer screening from multiple organizations are summarized in Table 3.39–43 Notably, the American Cancer Society now recommends HPV testing for primary screening beginning at 25 years of age. Table 4 summarizes HPV tests that are approved by the U.S. Food and Drug Administration (FDA) for use in women, all of which have similar sensitivities and specificities.44–49 There are no FDA-approved HPV tests for use in men or the oropharynx.
| Organization (year guideline released) | Age range (years) | Recommendation |
|---|---|---|
| ACS (2020) | 25 to 65 | Recommended: primary HPV testing every five years through 65 years of age; acceptable: cotesting every five years or cytology alone every three years if HPV testing is unavailable |
| Older than 65 | Discontinue screening if there is no history of CIN 2 or higher within the previous 25 years or there are adequate negative screening results over the previous 10 years | |
| ACS/ASCCP/ASCP (2012) | 21 to 29 | Cytology alone every three years |
| 30 to 65 | Recommended: cotesting every five years; acceptable: cytology alone every three years | |
| Older than 65 | No screening if there are adequate negative results on previous screening | |
| ACS/ASCCP/ASCP/Society of Gynecologic Oncology (2015; interim update) | Older than 25 | Primary high-risk HPV testing may be considered to replace current cytology-based approaches |
| American College of Obstetricians and Gynecologists (2016) | 21 to 29 | Cytology alone every three years |
| 30 to 65 | Recommended: cotesting every five years; acceptable: cytology alone every three years | |
| Older than 65 | No screening if there are adequate negative results on previous screening and no history of CIN 2 or higher | |
| U.S. Preventive Services Task Force (2018) | 21 to 29 | Cytology alone every three years |
| 30 to 65 | Cytology alone every three years, primary HPV testing every five years, or cotesting every five years | |
| Older than 65 | No screening if there are adequate negative results on previous screening and no history of CIN 2 or higher |
| Test* | Test type | No. of high-risk HPV types detected | Results reported | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Aptima HPV | RNA Amplification of E6 and E7 proteins | 14 | Positive or negative (no type-specific results) | 97.6 | 90.2 |
| Cervista HPV HR | DNA Amplification | 14 | Positive or negative (no type-specific results) | 89 | 91 |
| Cobas HPV† | DNA Amplification and genotyping of HPV-16 and HPV-18 | 14 | Pooled positive or negative for 12 high-risk types (no type-specific results); type-specific results for HPV-16 and HPV-18 | 97.3 | 84.5 |
| Hybrid Capture II | DNA Direct genome detection | 13 | Positive or negative (no type-specific results) | 97.5 | 84.3 |
The U.S. Preventive Services Task Force found insufficient evidence to recommend for or against oral cancer screening in asymptomatic individuals, and this finding is supported by the American Academy of Family Physicians.50,51 The American Dental Association recommends that dentists routinely perform visual and tactile examinations for oral and oropharyngeal carcinoma in all patients.52
Anal Pap testing has been considered in people at increased risk of anal cancer, including those with HIV, men who have sex with men, and individuals who have receptive anal intercourse.53,54 However, the Centers for Disease Control and Prevention has found insufficient data about the natural history of anal HPV infection to provide a recommendation for such screening.54 Abnormal findings on anal Pap smear and high-grade disease on cytology are common in these high-risk populations.54 Because cytology may underestimate the level of dysplasia in the anal intraepithelial neoplasia, if anal Pap smear is performed and findings are positive, anoscopy and biopsy should be performed to rule out anal carcinoma.55
Prevention
Limiting the number of sex partners, delaying first intercourse until a later age, and consistently using condoms reduce the risk of HPV infection.13,14 Smoking and alcohol cessation should be recommended to reduce the risk of HPV persistence and the development of HPV-related malignancies.15,16 Use of male and female condoms and dental dams may decrease the spread of HPV and should be considered for coital and noncoital sexual activity.56 However, vaccination is the primary recommended modality for prevention.57 HPV vaccinations are prophylactic and do not treat current disease or prevent progression.58 Vaccination has been demonstrated to reduce prevalence of vaccine-type HPV in females, anogenital warts, and precancerous cervical lesions.59–61
HPV vaccination is most effective when administered before the onset of sexual activity.57 The FDA has approved vaccination in children and adults between nine and 45 years of age, and the Advisory Committee on Immunization Practices recommends vaccination at 11 or 12 years of age, irrespective of the patient's sex.57,62 Catch-up vaccination is recommended for inadequately immunized individuals 13 to 26 years of age.57 A Cochrane review found that vaccinating females, with or without HPV exposure, between 15 and 26 years of age decreases the risk of CIN 2 and 3, with a number needed to treat of 39.63,64 Although vaccination is not routinely recommended in older individuals because most adults have been exposed to HPV, shared decision-making can guide administration in patients 27 to 45 years of age who have not been previously vaccinated.57
In immunocompetent individuals immunized before 15 years of age, two doses of vaccine given six to 12 months apart are indicated.57 In individuals immunized between 15 and 26 years of age and in individuals of any age who are immunocompromised, three doses given over six months are recommended.57 HPV vaccination is safe, and the only contraindications are known allergy to the vaccine and current pregnancy.57
There are three FDA-approved HPV vaccines: quadrivalent (Gardasil), bivalent (Cervarix), and nonavalent (Gardasil 9). However, only the nonavalent vaccine has been available for use in the United States since 2017.
The recombinant quadrivalent vaccine protects against HPV types 6, 11, 16, and 18 and reduces the incidence of cervical, vaginal, and vulvar intraepithelial lesions and adenocarcinoma in situ; penile intraepithelial lesions; and anal warts and intraepithelial lesions.62,65,66 The recombinant bivalent vaccine protects against HPV types 16 and 18 and reduces the incidence of cervical intraepithelial lesions.62,66 These two vaccines are available outside the United States, and data from four Nordic countries demonstrate that the quadrivalent vaccine is 100% effective against HPV-16 and HPV-18 for 12 years and is associated with more than 90% seropositivity for all four HPV types at 14 years.67
The nonavalent vaccine protects against the same HPV types as the quadrivalent vaccine, as well as five additional types: 31, 33, 45, 52, and 58.68 These nine HPV types are responsible for more than 90% of HPV-related carcinomas.57 The nonavalent vaccine reduces the incidence of cervical, vaginal, and vulvar intraepithelial lesions, adenocarcinoma in situ, and carcinoma; penile intraepithelial lesions and carcinoma; and anal warts, intraepithelial lesions, and carcinoma.62,66 The nonavalent vaccine has demonstrated a high level of immunogenicity (93% to 100%).69 On June 9, 2020, the FDA approved adding the prevention of head and neck cancers caused by HPV as an indication for the nonavalent HPV vaccine.70
This article updates a previous article on this topic by Juckett and Hartman-Adams.71
Data Sources: A PubMed search was conducted using search terms such as human papilloma virus, cervical cancer, anal cancer, oropharyngeal cancer, human papilloma virus vaccination, risk factors, and prevention. An Essential Evidence Plus search included relevant POEMs, Cochrane reviews, clinical decision rules, and a targeted PubMed search. Search dates: January 29, 2020; February 17, 2020; April 6, 2020; June 22 to 24, 2020; and March 12, 2021.
The opinions and assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Uniformed Services University of the Health Sciences or the Department of the Navy.
