
Am Fam Physician. 2021;104(6):598-608
Related Editorial: Counseling Parents and Adolescents About Marijuana
Related Letter to the Editor: Cannabis Use During Pregnancy
Patient information: See related handout on talking points about marijuana for parents and teens.
Author disclosure: No relevant financial affiliations.
Cannabis use in the United States is increasing annually in people of all ages. This increase is fueled by state-level legalization, decreased risk perception, and increased social acceptability. Cannabis and its active components, cannabinoids, have been studied for medical uses and marketed in many commercial forms. Cannabis can impair short-term memory, judgment, and coordination, and there is substantial evidence that it can adversely affect multiple organ systems. Cannabinoids have potential adverse drug interactions with commonly prescribed analgesic, psychotropic, and cardiovascular medications. Current evidence supports cannabinoid use only for a limited number of conditions (chemotherapy-induced nausea and vomiting, specific pain and spasticity syndromes, and certain forms of childhood epilepsy); thus, physicians recommending cannabinoids need to weigh the potential harms vs. perceived benefits. The U.S. Preventive Services Task Force recommends universal screening for unhealthy drug use, including cannabis, in adults 18 years and older. However, the American Academy of Family Physicians does not support this recommendation because of the lack of evidence of benefit in screening patients for unhealthy drug use, except for opioid use disorder. Treatment of cannabis use disorder is largely behavioral and requires a patient-centered, multifaceted approach with a focus on patient education. Pharmacotherapy for cannabis use disorder is limited and experimental. Harm reduction strategies and education about cannabis withdrawal syndrome should be provided to patients. Interpretation of urine drug testing for cannabis is challenging because of the persistence of metabolites for four to five days after a single use and for one month after chronic daily use.
Legalization of cannabis in 36 U.S. states and the District of Columbia has transformed a once illegal drug into an over-the-counter remedy for numerous ailments.1 Cannabis has become a multibillion-dollar industry with approximately 15% of U.S. adults reporting cannabis use in 2017.2 Cannabis risk perception among U.S. adolescents and adults has steadily decreased in the past two decades, accompanied by an annual increase in cannabis use among these populations.2–4 Between 2002 and 2012, the percentage of adults 18 to 29 years of age reporting cannabis use within the previous year doubled from 10.5% to 21.2%.5 The 2017 Monitoring the Future survey found that the annual prevalence of cannabis use for 12th graders was 37.1%.6

| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| Patients with a personal or family history of psychotic disorders should avoid cannabis because of an increased risk of psychosis.7,31,32 | A | Systematic review and consistent evidence from RCTs |
| Cannabis should be avoided in pregnant and lactating patients because of potential risks to the infant.34,35 | C | Expert opinion and consensus guideline |
| Patients with long-term opioid use should avoid concurrent cannabis use.38–40 | B | Evidence from RCTs showing potential for some harm and unclear benefits |
| Cannabinoids should be considered as a third-line option for neuropathic pain if benefits outweigh risks.41 | B | Consistent evidence from RCTs and systematic reviews |
| Cannabinoids should be considered as second-line therapy for chemotherapy-induced nausea and vomiting.42 | B | Consistent, moderate-quality evidence from RCTs |
| Patients with cannabis use disorder should be offered a combination of psychosocial interventions.46 | B | Systematic review |
Cannabis use disorder develops in 19.5% of lifetime users.7 Adolescents are two to four times more likely than adults to develop cannabis use disorder within two years of use.8 A 2017 national survey showed that 22% of Americans incorrectly believe marijuana is not addictive, and 29% strongly believe that its use can prevent health problems.9
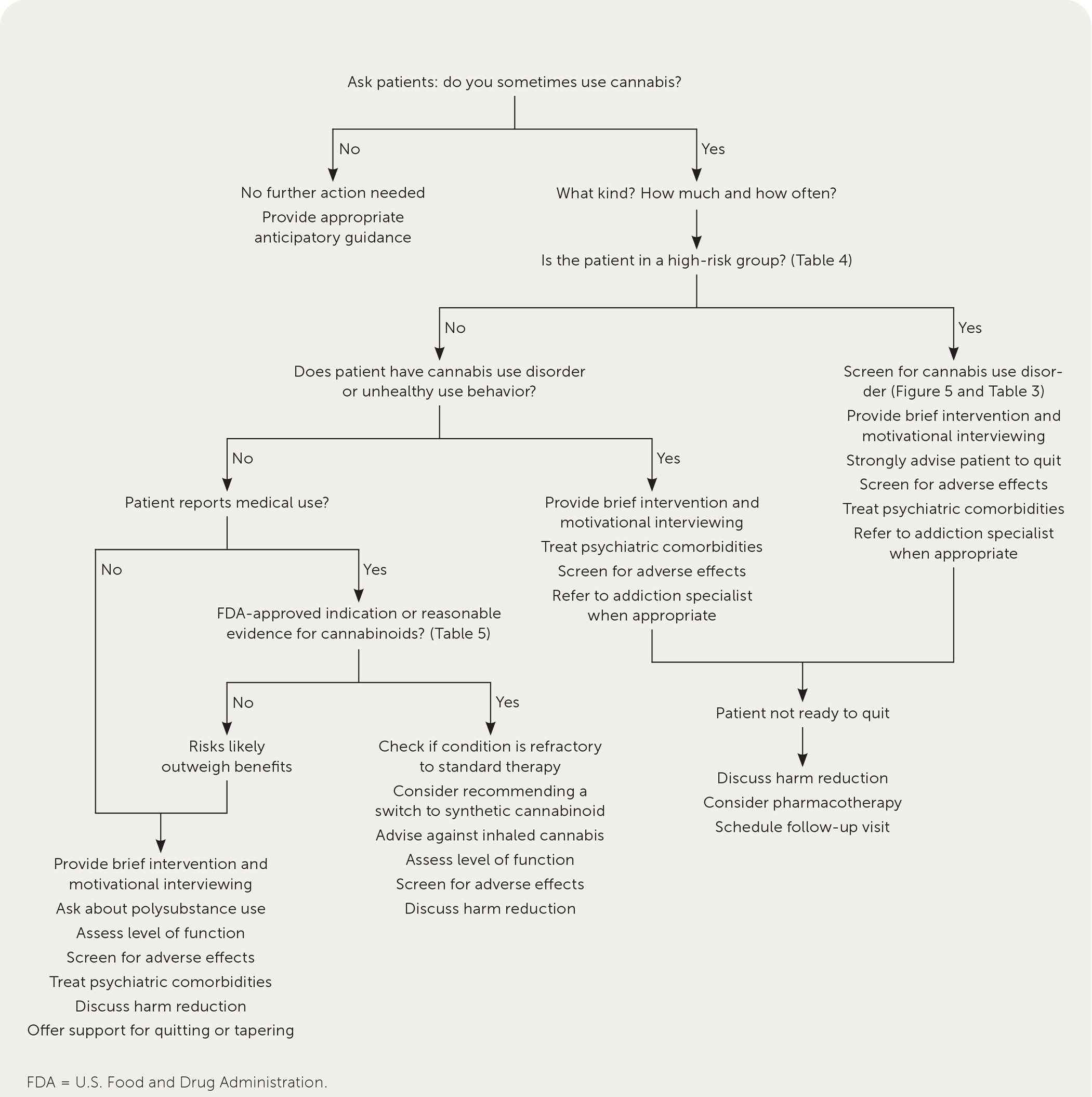
Figure 1 presents an approach to the management of patients who use cannabis.
Definitions
CANNABIS AND CANNABINOIDS
The Cannabis sativa plant contains more than 100 chemical structures called cannabinoids, including tetrahydrocannabinol (THC) and cannabidiol (CBD).10 THC has euphoric effects, whereas CBD is not psychoactive at typical doses.10,11 Marijuana and hemp are different strains of the same plant, the latter containing less than 0.4% of THC.10 Commercial marijuana commonly refers to the dried flowers (buds) and leaves of the C. sativa10 plant and is often cured in mason jars (Figure 2). The potency of THC in cannabis has been rising since the 1970s because of selective breeding. The average THC content of a cannabis joint rose from 1.5% in the 1970s to 8.9% in 2008 and 21% in 2018.12

Table 1 summarizes common types of cannabis products and methods of use.10,11 Smoking involves a bud or resin plus a device such as rolled paper, a pipe, or a bong. Vaping involves a bud, resin, or liquid solution placed in a heating device. Dabbing involves inhaling a potent concentrate high in THC. Edibles are available in many formulations (Figure 3).
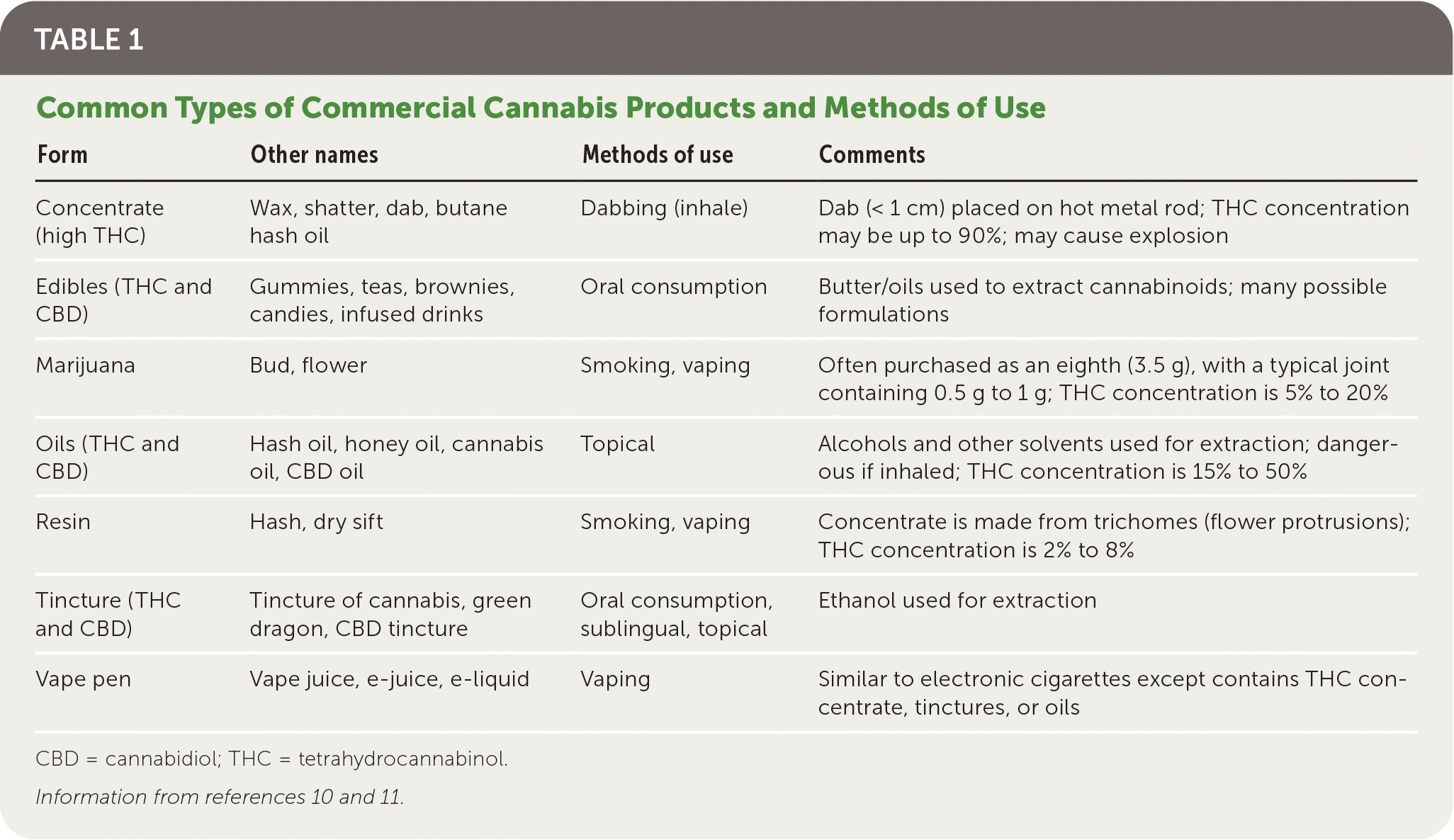
| Form | Other names | Methods of use | Comments |
|---|---|---|---|
| Concentrate (high THC) | Wax, shatter, dab, butane hash oil | Dabbing (inhale) | Dab (< 1 cm) placed on hot metal rod; THC concentration may be up to 90%; may cause explosion |
| Edibles (THC and CBD) | Gummies, teas, brownies, candies, infused drinks | Oral consumption | Butter/oils used to extract cannabinoids; many possible formulations |
| Marijuana | Bud, flower | Smoking, vaping | Often purchased as an eighth (3.5 g), with a typical joint containing 0.5 g to 1 g; THC concentration is 5% to 20% |
| Oils (THC and CBD) | Hash oil, honey oil, cannabis oil, CBD oil | Topical | Alcohols and other solvents used for extraction; dangerous if inhaled; THC concentration is 15% to 50% |
| Resin | Hash, dry sift | Smoking, vaping | Concentrate is made from trichomes (flower protrusions); THC concentration is 2% to 8% |
| Tincture (THC and CBD) | Tincture of cannabis, green dragon, CBD tincture | Oral consumption, sublingual, topical | Ethanol used for extraction |
| Vape pen | Vape juice, e-juice, e-liquid | Vaping | Similar to electronic cigarettes except contains THC concentrate, tinctures, or oils |

THE ENDOCANNABINOID SYSTEM
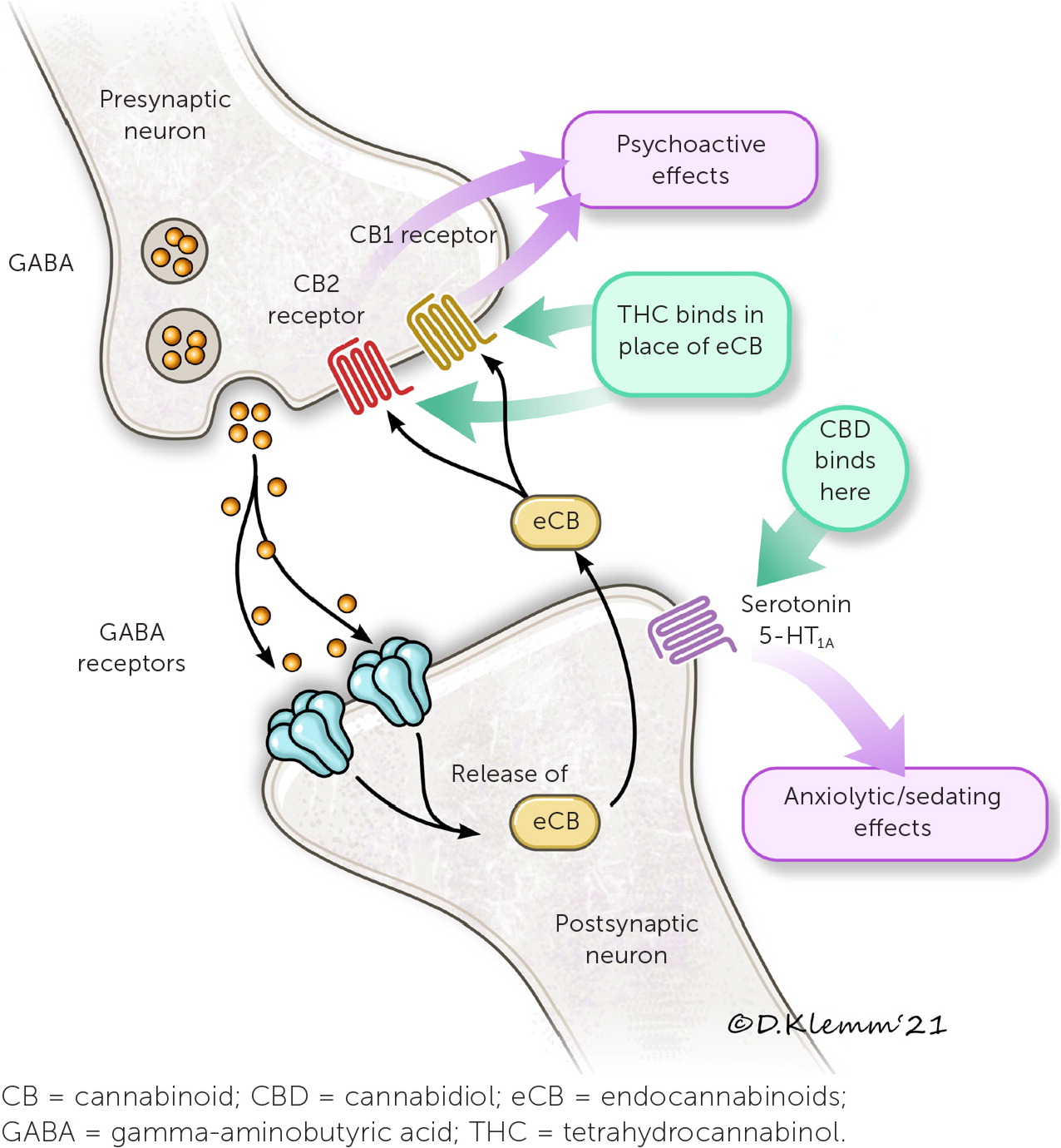
Cannabinoids act via the endocannabinoid system. The endocannabinoid system is involved in many physiologic processes with two main receptors: CB1 and CB2.10,13 Two endogenous cannabinoids, anandamide and 2-arachidonoylglycerol, bind these receptors. Gamma-aminobutyric acid and glutamate are involved in these neural pathways as inhibitory and excitatory neurotransmitters, respectively (Figure 4).10,13 THC mimics endogenous cannabinoids by stimulating CB1 and CB2 receptors in the central nervous system, whereas CBD stimulates serotonin 5-HT1A receptors.10,13
SYNTHETIC CANNABINOIDS
Three cannabinoid medications have been approved by the U.S. Food and Drug Administration (FDA). Dronabinol (Marinol) and nabilone (Cesamet) were approved in 1985 and 2006, respectively, for chemotherapy-induced nausea and vomiting.10 CBD (Epidiolex) was approved in 2018 for rare forms of childhood epilepsy.10,14 Nabiximols (Sativex) is approved in 28 countries, including the United Kingdom and Canada, but has not received FDA approval. Cannabinoid medications are summarized in (Table 2).10
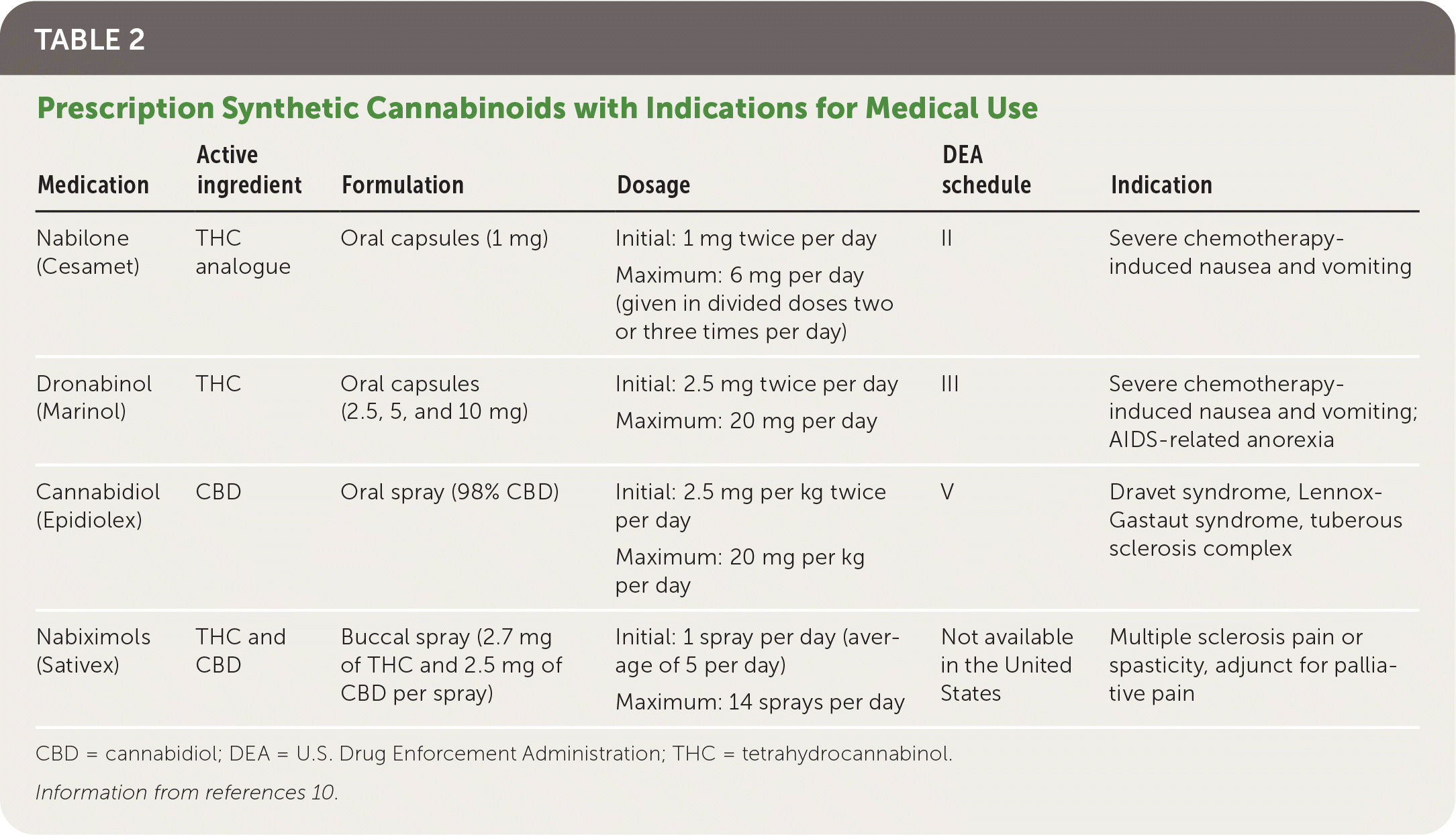
| Medication | Active ingredient | Formulation | Dosage | DEA schedule | Indication |
|---|---|---|---|---|---|
| Nabilone (Cesamet) | THC analogue | Oral capsules (1 mg) | Initial: 1 mg twice per day | II | Severe chemotherapy-induced nausea and vomiting |
| Maximum: 6 mg per day (given in divided doses two or three times per day) | |||||
| Dronabinol (Marinol) | THC | Oral capsules (2.5, 5, and 10 mg) | Initial: 2.5 mg twice per day | III | Severe chemotherapy-induced nausea and vomiting; AIDS-related anorexia |
| Maximum: 20 mg per day | |||||
| Cannabidiol (Epidiolex) | CBD | Oral spray (98% CBD) | Initial: 2.5 mg per kg twice per day | V | Dravet syndrome, Lennox-Gastaut syndrome, tuberous sclerosis complex |
| Maximum: 20 mg per kg per day | |||||
| Nabiximols (Sativex) | THC and CBD | Buccal spray (2.7 mg of THC and 2.5 mg of CBD per spray) | Initial: 1 spray per day (average of 5 per day) | Not available in the United States | Multiple sclerosis pain or spasticity, adjunct for palliative pain |
| Maximum: 14 sprays per day |
Several illegal synthetics, such as K2 (also called Spice), have more potent CB1 agonism than THC. Intoxication from illegal synthetics can be life-threatening, resulting in seizures, kidney injury, hypertension, and reduced cardiac perfusion.15 K2 is sold as a liquid that can be vaped or sprayed onto plant material that is smoked in a joint or pipe.
CANNABIS USE DISORDER
A validated screening tool such as the Cannabis Use Disorder Identification Test-Revised (CUDIT-R) can be used to screen patients for cannabis use disorder (Figure 5).16 A positive result on CUDIT-R screening has a 91% sensitivity and 90% specificity for detecting cannabis use disorder.16 Diagnosis of cannabis use disorder requires fulfillment of the Diagnostic and Statistical Manual of Mental Disorders, 5th ed., (DSM-5) criteria (Table 317).
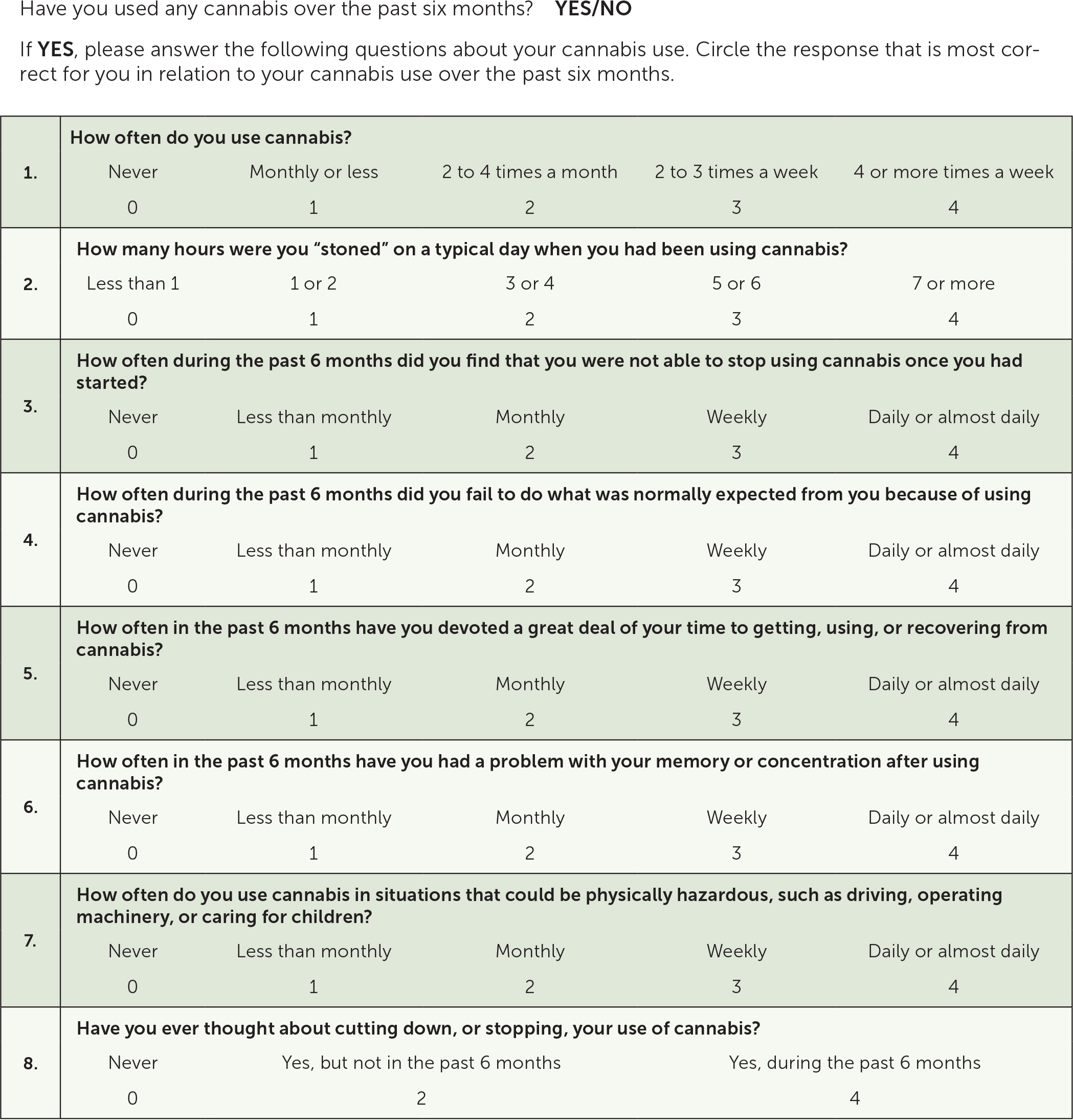

|
The U.S. Preventive Services Task Force recommends universal screening for unhealthy drug use in adults 18 years and older. However, the American Academy of Family Physicians does not support this recommendation because of the lack of evidence of benefit in screening patients for unhealthy drug use, except for opioid use disorder.18
Mechanism of Action
ABSORPTION AND METABOLISM
THC is highly lipophilic and crosses the blood-brain barrier. Cannabis inhalation produces a rapid-onset high (within five to 10 minutes) that lasts two to four hours.11 The high from edibles, by contrast, has a slower onset (60 to 180 minutes), is less intense, and lasts longer (about six to eight hours).11 Cannabis metabolism involves hepatic cytochrome P450 3A4, 2C9, and 2C19 enzymes, so medications that are inhibitors, inducers, or substrates of these enzymes may alter drug levels.19
DRUG INTERACTIONS
Cannabinoids can potentially interact with several drug classes, such as commonly prescribed analgesic, psychotropic, and cardiovascular medications. However, the effects are not well-characterized. Both THC and CBD may alter levels of certain opioids, benzodiazepines, statins, anti-depressants, and anticoagulants.19 THC may increase the effects of central nervous system depressants (e.g., alcohol, opioids, benzodiazepines), resulting in added impairment.19 Although cannabinoids are not included in the 2019 American Geriatrics Society Beers Criteria,20 physicians should recognize the risks of polypharmacy in older patients who are using cannabinoids with two or more other psychoactive drugs included in the Beers Criteria.
Common Syndromes
Cannabinoid hyperemesis syndrome is more common in males and presents with cyclic vomiting, diffuse abdominal pain, and relief with hot showers.23 Traditional antiemetics are often ineffective for cannabinoid hyperemesis syndrome, but topical capsaicin applied to the abdomen, back, or arms and dopamine antagonists might help reduce symptoms.23
Cannabis withdrawal syndrome occurs in about 47% of regular users.24 It presents with anxiety as early as four hours after cessation, with other symptoms manifesting one to two days later and lasting up to three to four weeks.22 Symptoms vary depending on the dose and duration of cannabis use but are not life-threatening.22,24 Self-management with exercise, relaxation techniques, and over-the-counter analgesics can be recommended to patients. Off-label use of synthetic cannabinoids, gabapentin (Neurontin), quetiapine (Seroquel), or mirtazapine (Remeron) can be considered for moderate to severe cases.22
Cannabis withdrawal syndrome is diagnosed when more than three of the following DSM-5 criteria are present within approximately seven days of reduced use:(1) irritability, anger, or aggression; (2) nervousness or anxiety; (3) sleep difficulty (e.g., insomnia, disturbing dreams); (4) decreased appetite or weight loss; (5) restlessness; (6) depressed mood; (7) at least one physical symptom causing significant discomfort (abdominal pain, tremors, diaphoresis, fevers, chills, headache).17
Health Risks of Cannabis
Table 4 summarizes patient groups at highest risk of cannabis-related harm. Cannabis can impair short-term memory, judgment, and coordination, resulting in a three-to sevenfold increased risk of motor vehicle crashes.8 Cannabis appears to be a neurotoxin affecting brain development, and long-term studies demonstrate associations with lower IQ, poor educational outcomes, impaired executive function, and avolition.25,26 Impairments appear to affect multiple domains irrespective of social status; however, a meta-analysis of 69 studies (n = 8,727) showed that cognitive impairment can diminish after 72 hours of abstinence.25–27 Adverse effects on young, developing brains appear to be driven by heavy use.27
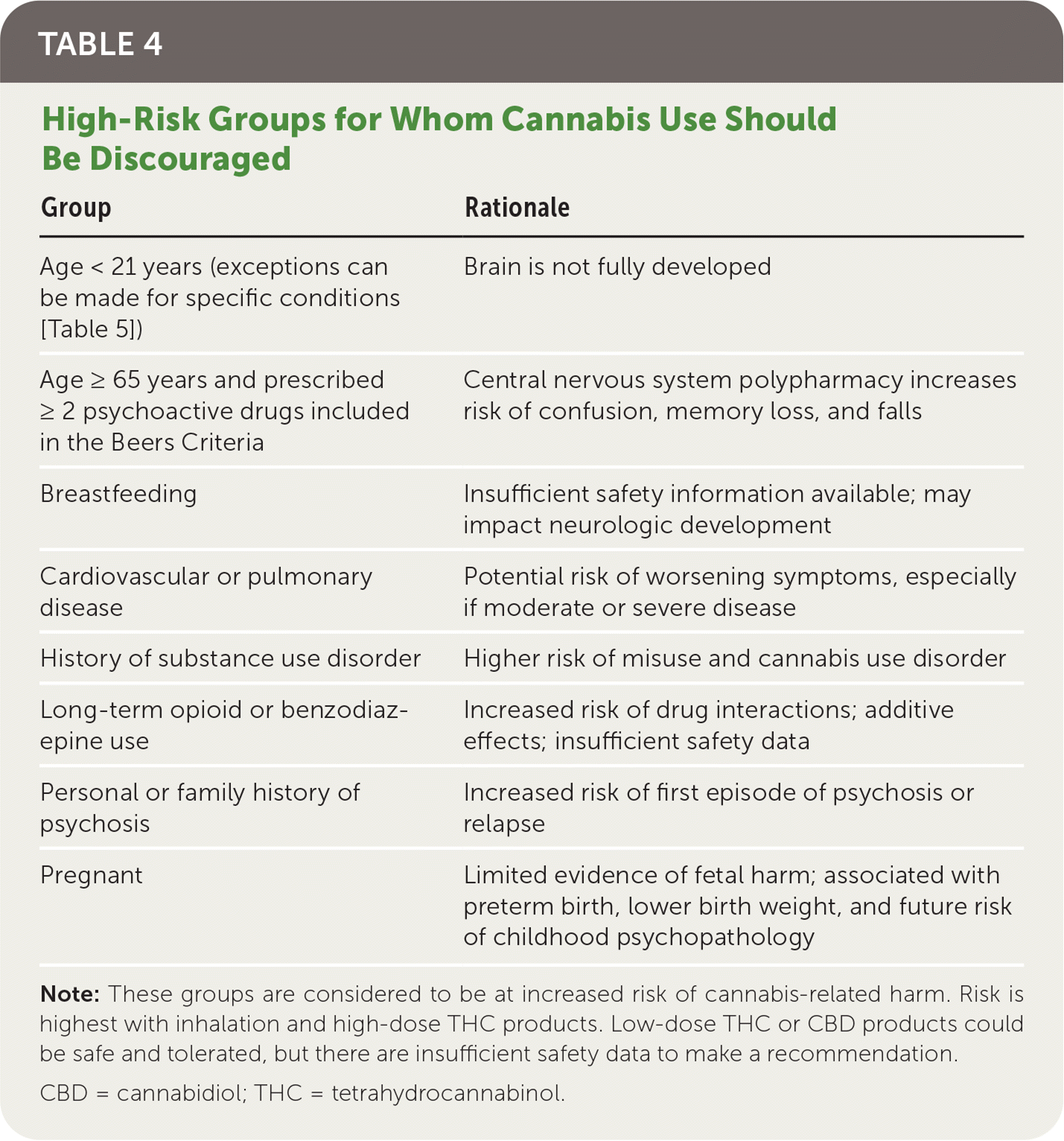
| Group | Rationale |
|---|---|
| Age < 21 years (exceptions can be made for specific conditions [Table 5]) | Brain is not fully developed |
| Age ≥ 65 years and prescribed ≥ 2 psychoactive drugs included in the Beers Criteria | Central nervous system polypharmacy increases risk of confusion, memory loss, and falls |
| Breastfeeding | Insufficient safety information available; may impact neurologic development |
| Cardiovascular or pulmonary disease | Potential risk of worsening symptoms, especially if moderate or severe disease |
| History of substance use disorder | Higher risk of misuse and cannabis use disorder |
| Long-term opioid or benzodiazepine use | Increased risk of drug interactions; additive effects; insufficient safety data |
| Personal or family history of psychosis | Increased risk of first episode of psychosis or relapse |
| Pregnant | Limited evidence of fetal harm; associated with preterm birth, lower birth weight, and future risk of childhood psychopathology |
Psychiatric comorbidity is common in cannabis users. Long-term cannabis use increases the risk of future anxiety disorders in cohort studies, even with cessation.28 Adolescent use is associated with increased risk of self-harm, suicidality, and all-cause mortality.29 A large meta-analysis (n = 296,815) found moderate associations between cannabis use and risk of physical violence (odds ratio = 2.62) after adjusting for socioeconomic status and polysubstance use.30
Cannabis use is a modifiable risk factor for psychosis, especially in those who are genetically predisposed.7 One observational study found a four- to fivefold increased risk of a first episode of psychosis in people who use high-potency (more than 10% THC) cannabis daily.31 A systematic review of 24 studies of patients with schizophrenia (n = 16,565) found that cannabis users had higher relapse rates, longer hospitalizations, and more positive symptoms compared with those who do not use cannabis.32
Other risks of long-term cannabis use include male infertility, symptoms of chronic bronchitis, and cannabis use disorder.7,8,33 THC crosses the placenta, and low-strength evidence links prenatal marijuana use to preterm birth, lower birth weight, and future risk of childhood psychopathology.34 THC can be present in breast milk up to six days after use, but data about lactation safety are limited.11 In 2019, the surgeon general issued a warning against cannabis use by people who are pregnant or lactating.35
Cannabis and Opioids
Evidence of opioid-sparing effects of cannabis for pain management is mixed. One observational study (n = 1,321) showed that low-frequency cannabis use was associated with lower opioid use.36 Ecological studies have shown small reductions in opioid prescriptions after cannabis legalization, but they have not shown fewer opioid deaths.7 Such trends could be driven not only by cannabis legalization, but by various measures to curb the opioid epidemic, resulting in fewer opioid prescriptions in recent years.7,37
A prospective study of nonmedical opioid users (n = 211) found an almost twofold increased risk of opioid use on days when cannabis was used, regardless of pain severity.38 Another prospective cohort study (n = 1,514) of patients with chronic pain who were using opioids found cannabis use to be associated with greater pain and lower self-efficacy.39 A systematic review of nine studies found little evidence that cannabinoids reduce opioid use, despite consistent evidence of a synergistic effect.40 Overall, low-strength evidence supports recommending against concurrent opioid and cannabis use.
Evidence for Medical Use
Cannabinoid trials have included both C. sativa (buds/resin or extracts) and synthetic cannabinoids. Study limitations include heterogeneity of products, high risk of bias, and limited applicability to vulnerable populations.41,42 A meta-analysis of 104 studies (n = 9,958) showed that people using cannabinoids were more likely than those using placebo to have a 30% reduction in chronic pain (number needed to treat = 24; 95% CI, 15 to 62; number needed to harm = 6; 95% CI, 5 to 8) with no significant evidence for a 50% pain reduction threshold.42
Cannabinoids are more effective compared with placebo for chemotherapy-induced nausea and vomiting, specific pain and spasticity syndromes, and certain forms of childhood epilepsy (Table 5).41,42 A review of 31 systematic reviews recommended cannabinoids as a third-line option for neuropathic or palliative pain and a second-line option for chemotherapy-induced nausea and vomiting and multiple sclerosis spasticity, based on stronger evidence for standard therapies.41 The review also showed that the patients taking cannabinoids were three times more likely to withdraw because of adverse effects. There is no FDA-approved indication for inhaled cannabis.
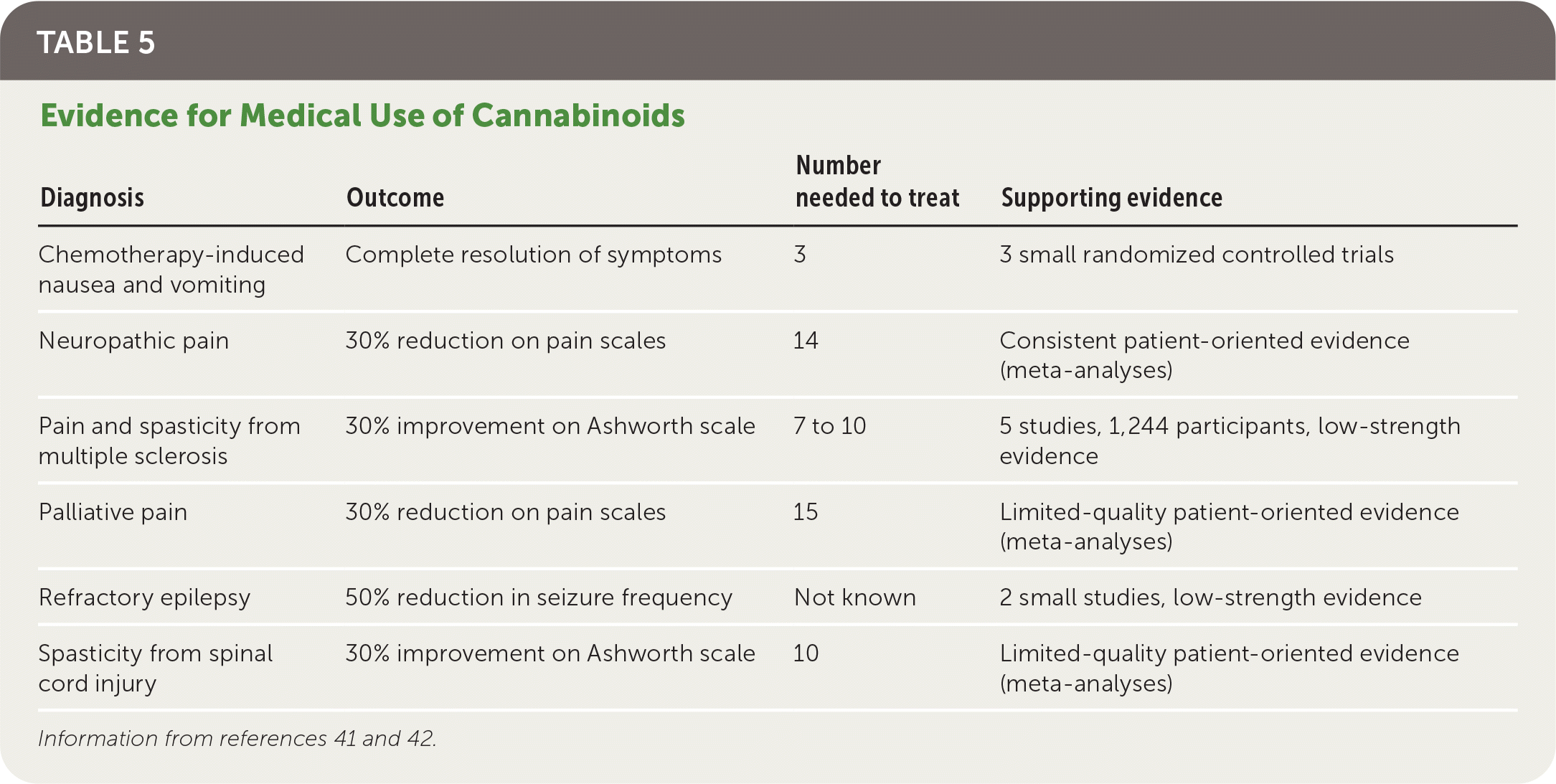
| Diagnosis | Outcome | Number needed to treat | Supporting evidence |
|---|---|---|---|
| Chemotherapy-induced nausea and vomiting | Complete resolution of symptoms | 3 | 3 small randomized controlled trials |
| Neuropathic pain | 30% reduction on pain scales | 14 | Consistent patient-oriented evidence (meta-analyses) |
| Pain and spasticity from multiple sclerosis | 30% improvement on Ashworth scale | 7 to 10 | 5 studies, 1,244 participants, low-strength evidence |
| Palliative pain | 30% reduction on pain scales | 15 | Limited-quality patient-oriented evidence (meta-analyses) |
| Refractory epilepsy | 50% reduction in seizure frequency | Not known | 2 small studies, low-strength evidence |
| Spasticity from spinal cord injury | 30% improvement on Ashworth scale | 10 | Limited-quality patient-oriented evidence (meta-analyses) |
There is insufficient evidence to recommend cannabinoids for patients with neurodegenerative and movement disorders; however, they appear to be well-tolerated in these patients at low doses.43 Although cannabinoids can mitigate symptoms of multiple sclerosis and Parkinson disease, their benefits should be weighed against the risk of adverse effects.43 Cannabinoids have been studied for treatment of mood and anxiety disorders and were found to temporarily reduce symptoms without producing remission.44
Treatment of Cannabis Use Disorder
Psychosocial interventions are the cornerstone of treatment for cannabis use disorder, and studies generally measure abstinence at six to 12 months. Brief counseling by primary care physicians has shown benefit for adolescents and nondaily users.45 Cognitive behavior therapy and motivational enhancement therapy are equally effective for reducing mean days of cannabis use, and maximal effectiveness occurs when they are used in combination.46 Cognitive behavior therapy and motivational enhancement therapy can be offered in groups or individually, and adding contingency management increases abstinent days.46
Pharmacotherapy for cannabis use disorder, although limited and experimental, can improve withdrawal and reduce cannabis use. Small studies have shown reduced cravings and cannabis use with gabapentin in adults and acetylcysteine in adolescents.47,48 Small trials of synthetic cannabinoids have shown little benefit.47 One small randomized controlled trial (n = 48) of high-dose CBD has shown promise as a substitution therapy, but further study is needed.49 Mirtazapine and quetiapine may modestly reduce cannabis use and withdrawal symptoms.22
Harm reduction strategies should be discussed with patients who use cannabis. These may include choosing products with low THC, avoiding daily use, reducing inhalation (to one puff every 15 minutes), purchasing from legal dispensaries (which have to comply with state regulations for contaminant testing, labeling, and dosing), and gradual tapering. Tapering should include education about cannabis withdrawal syndrome and a treatment plan for symptoms.
Drug Testing
Modern forms of urine drug screening check for the inactive metabolite 11-nor-9-carboxy-THC (THCCOOH) but offer little information about amount or time since last use.10,50 Urine tests can be positive for THCCOOH for four to five days after a single use of cannabis and for one month after chronic daily use.10,50 Passive exposure from secondhand smoke in poorly ventilated spaces may cause a transient positive result on urine drug screening for less than 24 hours.51 Confirmation of THCCOOH concentration (with liquid or gas chromatography and mass spectrometry) and comparing creatinine-normalized THCCOOH over time are diagnostic standards in urine drug screening.10,50
Data Sources: A PubMed search was completed in Clinical Queries using the key terms cannabis, marijuana, and THC with diagnosis, withdrawal, and treatment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. We also searched the Agency for Healthcare Research and Quality evidence reports, Clinical Evidence, the Cochrane database, Essential Evidence Plus, the Institute for Clinical Systems Improvement, and DynaMed. Search dates: April 21, 2021, and August 14, 2021.
