
Am Fam Physician. 2021;104(6):647-648
Author disclosure: No relevant financial affiliations.
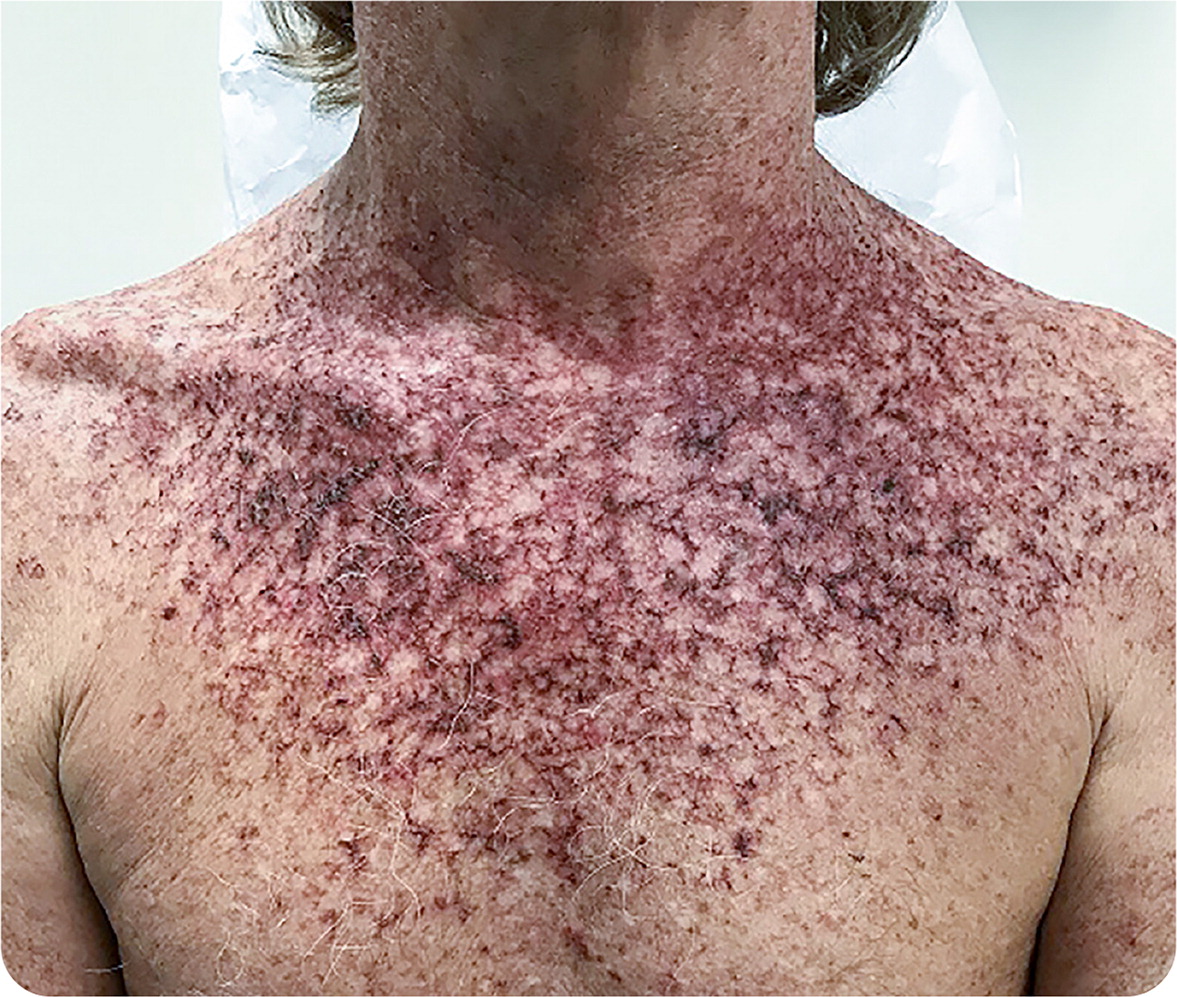
A 47-year-old man presented for a follow-up appointment after seeking medical attention for maculopapular lesions and scaling on sun-exposed areas of his chest and shoulders. The lesions had been present for more than one year. He was prescribed a course of a topical medication applied twice daily and subsequently developed erythema and scaling (Figure 1). The patient had no fever, drainage, warmth, or other indications of infection. He was a surfer and had a history of extensive sun exposure.
Question
These skin changes were diagnosed as an anticipated response to medication. Which one of the following medications is most likely the cause?
A. Fluorouracil 5% cream.
B. Methotrexate, 5 mg.
C. Neomycin ointment.
D. Tacrolimus 0.1% ointment.
E. Triamcinolone 0.1% cream.
Discussion
The correct answer is A: fluorouracil 5% cream. This avid surfer had extensive sun exposure, which led to multiple actinic keratoses that were treated with fluorouracil 5%, applied twice daily for four weeks.
Fluorouracil actively targets precancerous cells. Figure 1 illustrates a common reaction typically seen by week 3 of treatment. Sun exposure during treatment or applying the medication too thickly can lead to a robust reaction with potential for scarring. If this excessive response occurs, therapy may be halted for two or three days then restarted to reach the goal of two to four cumulative weeks of twice-daily application. Liberal use of moisturizer between applications can help alleviate irritation or pain. For thicker skin, longer duration of therapy or repeated treatment cycles may be required to effectively treat actinic keratoses.
Methotrexate may cause a phototoxic skin eruption. Many oral drugs, including methotrexate, hydrochlorothiazide, amiodarone, furosemide (Lasix), naproxen, piroxicam (Feldene), doxycycline, griseofulvin, promethazine, voriconazole (Vfend), and fenofibrate (Tricor), have photosensitizing effects that can cause phototoxic skin eruptions in sun-exposed areas.1 When evaluating a skin eruption in this pattern, a comprehensive medication history is necessary.
Neomycin ointment, which is indicated for the treatment of localized infections, can cause allergic contact dermatitis, especially in those older than 60 years.2 Additional risk factors for topical antibiotic allergies include wounds in postoperative settings, long-standing eczematous processes, chronic venous insufficiency, chronic otitis externa, and occupational exposure to topical antibiotics.2 Neomycin allergy may occur after the medication is used on inflamed skin for at least one week and may mimic signs of infection.2 Allergic contact dermatitis is characterized by a red, inflamed, weeping skin eruption, whereas irritant contact dermatitis often appears dry and scaly.
Tacrolimus 0.1% ointment (Protopic), a calcineurin inhibitor, is used to treat vitiligo, eczema, and irritant contact dermatitis. However, it carries a U.S. Food and Drug Administration (FDA) boxed warning because of the potential risk of malignancy, and the only FDA-approved indication is for atopic dermatitis as a second-line agent.3 Tacrolimus 0.03% ointment is approved for children older than two years. Apart from the boxed warning, which should be reviewed with parents, it avoids the thinning of skin and pigment changes that often occur with long-term topical corticosteroid use.3 Rarely patients experience a burning sensation or facial flush with alcohol consumption when using tacrolimus.4 This medication is not used to treat actinic keratoses.
Triamcinolone 0.1% cream is a topical corticosteroid and not appropriate for treatment of actinic keratoses. Triamcinolone cream may be used for two weeks to treat contact dermatitis because it calms inflammation and associated pruritus. Occasional allergic reactions to a topical corticosteroid may occur. Steroids are divided into four classes; if a patient has an allergy to one class, careful consideration should be given before prescribing other classes.5,6 A quick reference guide is available.6
The opinions expressed belong solely to the author. They should neither be interpreted as representative of nor endorsed by the U.S. Navy, the Department of Defense, or any other federal government agency.
