
Am Fam Physician. 2019;99(7):online
Author disclosure: No relevant financial affiliations.
Details for This Review
Study Population: Females 15 to 45 years of age who received human papillomavirus (HPV) vaccines (monovalent, bivalent [Cervarix], and quadrivalent [Gardasil])
Efficacy End Points: Rates of high-grade precancerous cervical lesions (cervical intraepithelial neoplasia [CIN] grade 2 or 3) and adenocarcinoma in situ in young women
Harm End Points: Adverse effects at site of administration, including local pain, swelling, redness, and itching; systemic effects, mortality, and effects related to miscarriage
Narrative: Cervical cancer mortality has decreased in incidence since the adoption of routine screening with the Papanicolaou smear. Despite screening, cervical cancer was responsible for 266,000 deaths worldwide in 2012.1 Precancerous cervical lesions and cervical cancer are caused by HPV in 99% of cases.2 Persistent infection with certain types of HPV, referred to as high-risk HPV (hrHPV) strains, are more likely to lead to cervical cancer than other types. Of the hrHPV types, HPV types 16 and 18 are associated with 70% of all cervical cancers. Several vaccinations targeted against HPV have been developed with the goal of reducing cervical cancer and mortality.
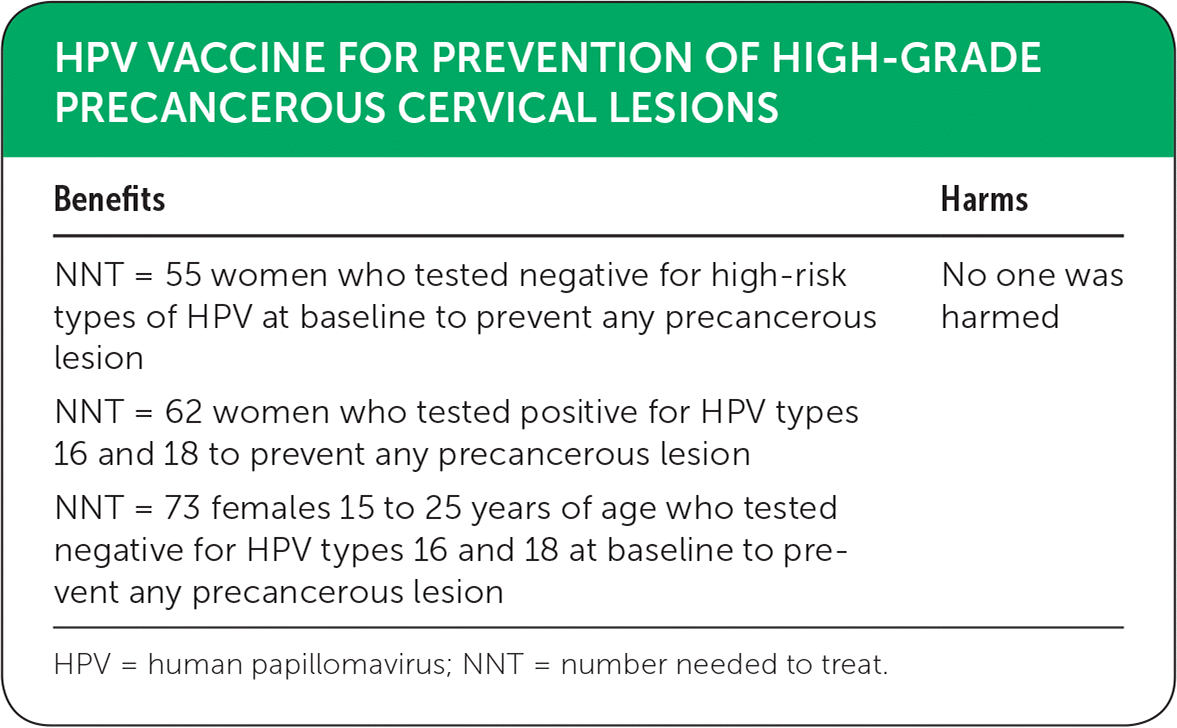
The systematic Cochrane review included 26 randomized controlled trials (N = 73,428) evaluating the efficacy and safety of HPV vaccines compared with placebo.3 Results were separated into three groups based on exposure to HPV at enrollment: hrHPV-negative (15 to 25 years of age); HPV types 16- or 18-negative; or undisclosed HPV status. Women who were negative for any hrHPV appeared to receive the greatest benefit from vaccination, with a number needed to treat (NNT) of 55 for preventing any precancerous lesions (risk reduced from 287 to 106 per 10,000; relative risk [RR] = 0.37; 95% CI, 0.25 to 0.55). For lesions associated with HPV types 16 or 18 in this group, the NNT was 62 (risk reduced from 164 to 2 per 10,000; RR = 0.01; 95% CI, 0 to 0.05). In women who tested negative for HPV types 16 and 18 at enrollment, the results differed based on age. Females 15 to 25 years of age had an NNT of 73 for preventing any precancerous lesion (risk reduced from 231 to 95 per 10,000); women older than 25 years demonstrated an NNT of 322 for this outcome (risk reduced from 45 to 14 per 10,000). A difference in results based on age was also noted in the undisclosed HPV status group. The NNT for preventing any precancerous lesion was 60 for females 15 to 25 years of age (risk reduced from 559 to 391 per 10,000), whereas the precancer rates were similar between the vaccinated and unvaccinated groups in women older than 25 years (rate of 356 and 343 per 10,000, respectively).
| Benefits | Harms |
|---|---|
| NNT = 55 women who tested negative for high-risk types of HPV at baseline to prevent any precancerous lesion | No one was harmed |
| NNT = 62 women who tested positive for HPV types 16 and 18 to prevent any precancerous lesion | |
| NNT = 73 females 15 to 25 years of age who tested negative for HPV types 16 and 18 at baseline to prevent any precancerous lesion |
Adverse outcomes were similar in the control and vaccination groups. Mortality rate was 11 per 10,000 in the control group vs. 14 per 10,000 in the vaccine group. The deaths were associated with older women but were determined not to be related to the vaccine.
These data suggest the benefits of vaccination for reducing precancerous lesions outweigh the potential harms. Although cancer rates were not assessed, it can reasonably be assumed that lower rates of precancerous lesions will lead to lower cancer rates in the long term. Overall, less benefit was seen in older women and women unselected for HPV status, because these groups were more likely to have a history of HPV exposure. Because the greatest benefits were in younger women (15 to 25 years of age) who were documented as HPV-negative at enrollment, this review demonstrates the importance of vaccination before HPV exposure (e.g., onset of sexual activity).
Caveats: Three different vaccines were used in the study: monovalent, bivalent, and quadrivalent. The bivalent vaccine appears to have greater protection against CIN 3+ with an RR of 0.08 compared with the quadrivalent vaccine, with an RR of 0.54 compared with placebo. This was attributed to the difference of HPV prevalence in different populations, as well as different protocols and assays used at different sites. Additionally, progression from dysplasia to cancer occurs over a time frame of many years. The short duration of median follow-up in the trials (3.5 to 8 years) makes the results vulnerable to lag time bias, which is demonstrated by the overall small reduction in adenocarcinoma in situ (9 to 0 per 10,000; RR = 0.10; 95% CI, 0.01 to 0.82). Lastly, this review incorporated studies from multiple countries, which could have resulted in varying standards of care with regard to screening practices.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the United States Army medical corps or the U.S. Army at large.
