
Am Fam Physician. 2021;103(10):590-596
Published online February 2, 2021.
Author disclosure: No relevant financial affiliations.
More than 5 million patients in the United States are admitted to intensive care units (ICUs) annually, and an increasing percentage of patients treated in the ICU survive to hospital discharge. Because these patients require follow-up in the outpatient setting, family physicians should be prepared to provide ongoing care and screening for post-ICU complications. Risk factors for complications after ICU discharge include previous ICU admissions, preexisting mental illness, greater number of comorbidities, and prolonged mechanical ventilation or higher opioid exposure while in the ICU. Early nutritional support and mobilization in the ICU decrease the risk of complications. After ICU discharge, patients should be screened for depression, anxiety, insomnia, and cognitive impairment using standardized screening tools. Physicians should also inquire about weakness, fatigue, neuropathy, and functional impairment and perform a targeted physical examination and laboratory evaluation as indicated; treatment depends on the underlying cause. Exercise regimens are beneficial for reducing several post-ICU complications. Patients who were treated for COVID-19 in the ICU may require additional instruction on reducing the risk of virus transmission. Telemedicine and telerehabilitation allow patients with COVID-19 to receive effective care without increasing exposure risk in communities, hospitals, and medical offices.
More than 5 million patients in the United States are admitted to intensive care units (ICUs) annually.1 Mortality rates among these patients decreased by 35% between 1988 and 2012, despite increasing illness severity and increasing patient age; current mortality rates are estimated at 10% to 29%.1 Because a greater number of patients are surviving to ICU discharge and require follow-up in the outpatient setting, family physicians should be prepared to provide ongoing care and screening for post-ICU complications.
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| Patients should be screened for weakness after ICU discharge and referred for physical rehabilitation when appropriate.5–8 | B | Small randomized controlled trials and professional guideline |
| Patients should be screened for cognitive impairment after ICU discharge.20–22 | C | Observational and retrospective cohort studies |
| Patients should be screened for depression, anxiety,and posttraumatic stress disorder after ICU discharge.5,24,25 | C | Prospective cohort studies |
| Partners and family members of patients discharged from the ICU should be screened for depression, anxiety, and posttraumatic stress disorder.27,28 | C | Prospective cohort studies |
With no universally accepted guidelines, the timing and frequency of post-ICU follow-up should be individualized and based on the patient's comorbidities and severity of illness. Although about 20 medical centers in the United States have post-ICU transition clinics,2 there is no evidence that such programs decrease readmission or mortality rates.3
Although the term post-ICU syndrome has been used to describe the various complications reported in ICU survivors (Table 14 ), there is no universally accepted definition for such a syndrome. Family physicians must be familiar with the complications that can occur after discharge (e.g., physical and psychological impairments), including in patients recovering from COVID-19.
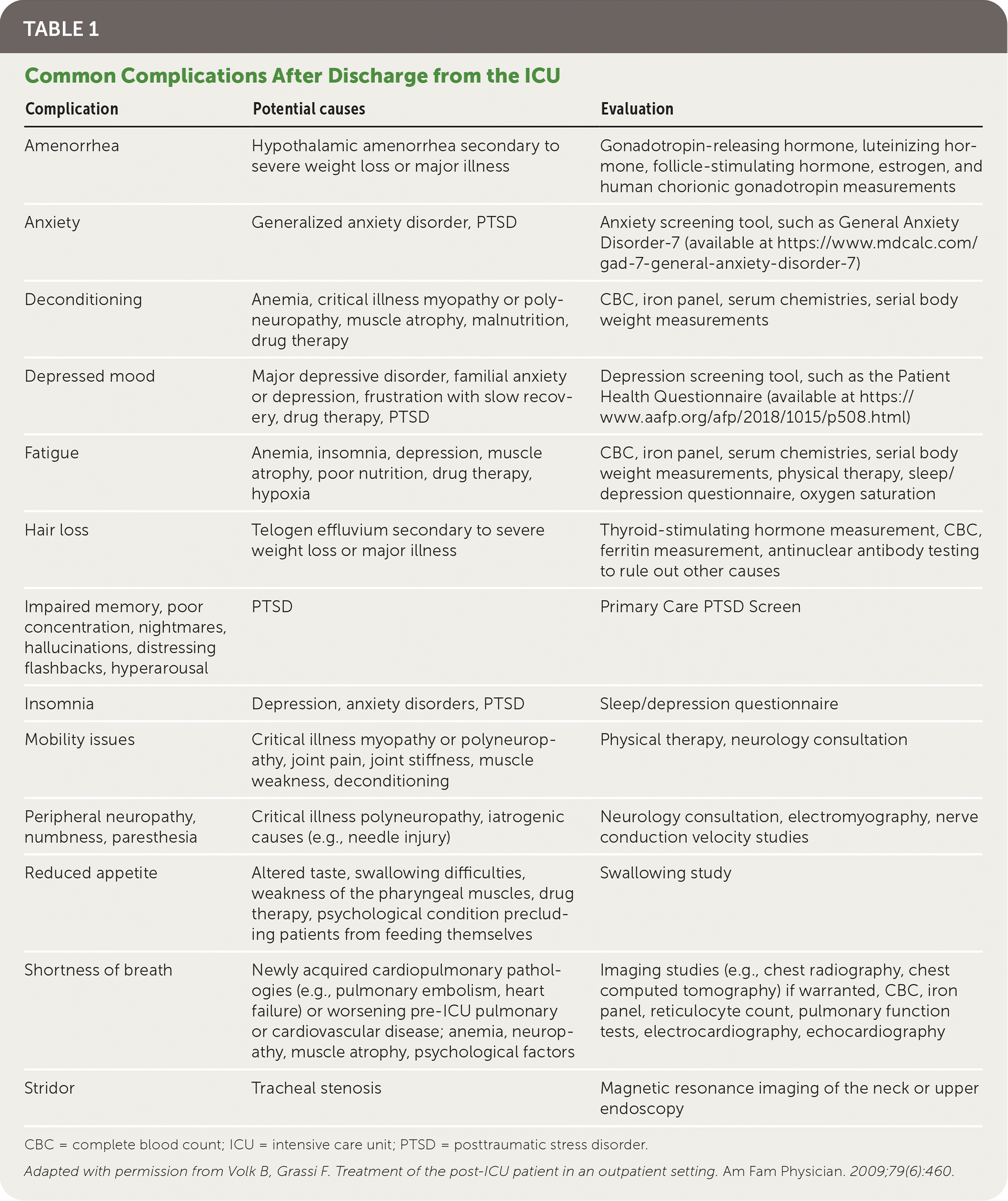
| Complication | Potential causes | Evaluation |
|---|---|---|
| Amenorrhea | Hypothalamic amenorrhea secondary to severe weight loss or major illness | Gonadotropin-releasing hormone, luteinizing hormone, follicle-stimulating hormone, estrogen, and human chorionic gonadotropin measurements |
| Anxiety | Generalized anxiety disorder, PTSD | Anxiety screening tool, such as General Anxiety Disorder-7 (available at https://www.mdcalc.com/gad-7-general-anxiety-disorder-7) |
| Deconditioning | Anemia, critical illness myopathy or poly-neuropathy, muscle atrophy, malnutrition, drug therapy | CBC, iron panel, serum chemistries, serial body weight measurements |
| Depressed mood | Major depressive disorder, familial anxiety or depression, frustration with slow recovery, drug therapy, PTSD | Depression screening tool, such as the Patient Health Questionnaire (available at https://www.aafp.org/afp/2018/1015/p508.html) |
| Fatigue | Anemia, insomnia, depression, muscle atrophy, poor nutrition, drug therapy, hypoxia | CBC, iron panel, serum chemistries, serial body weight measurements, physical therapy, sleep/depression questionnaire, oxygen saturation |
| Hair loss | Telogen effluvium secondary to severe weight loss or major illness | Thyroid-stimulating hormone measurement, CBC, ferritin measurement, antinuclear antibody testing to rule out other causes |
| Impaired memory, poor concentration, nightmares, hallucinations, distressing flashbacks, hyperarousal | PTSD | Primary Care PTSD Screen |
| Insomnia | Depression, anxiety disorders, PTSD | Sleep/depression questionnaire |
| Mobility issues | Critical illness myopathy or polyneuropathy, joint pain, joint stiffness, muscle weakness, deconditioning | Physical therapy, neurology consultation |
| Peripheral neuropathy, numbness, paresthesia | Critical illness polyneuropathy, iatrogenic causes (e.g., needle injury) | Neurology consultation, electromyography, nerve conduction velocity studies |
| Reduced appetite | Altered taste, swallowing difficulties, weakness of the pharyngeal muscles, drug therapy, psychological condition precluding patients from feeding themselves | Swallowing study |
| Shortness of breath | Newly acquired cardiopulmonary pathologies (e.g., pulmonary embolism, heart failure) or worsening pre-ICU pulmonary or cardiovascular disease; anemia, neuropathy, muscle atrophy, psychological factors | Imaging studies (e.g., chest radiography, chest computed tomography) if warranted, CBC, iron panel, reticulocyte count, pulmonary function tests, electrocardiography, echocardiography |
| Stridor | Tracheal stenosis | Magnetic resonance imaging of the neck or upper endoscopy |
Physical Considerations
DECONDITIONING
One year after ICU discharge, more than 20% of patients without functional limitations before admission experience some difficulty in completing activities of daily living.5 The National Institute for Health and Care Excellence recommends reassessing patients' physical function two to three months following discharge.6 Exercise and self-help rehabilitation programs improve muscle function and cardiopulmonary function after critical illness.7,8 Patients with deconditioning should be instructed on starting a home exercise regimen or referred to physical therapy.
CRITICAL ILLNESS POLYNEUROPATHY
Patients treated in the ICU are at risk of critical illness (or ICU-acquired) polyneuropathy, a disease of the peripheral nerves secondary to axonal degeneration. This condition is caused by complex and poorly understood processes that include microcirculatory abnormalities, metabolic derangements, and other factors.9–11 Critical illness polyneuropathy is diagnosed by the presence of limb weakness and unexplained difficulty in weaning from mechanical ventilation. Sepsis, prolonged mechanical ventilation, hyperglycemia, and multiorgan failure increase the risk of critical illness polyneuropathy.9,10
Although nerve function usually begins to improve when major medical issues are resolved, weakness and numbness may persist in severe cases. There is little evidence for physical rehabilitation or pharmacologic interventions in the treatment of critical illness polyneuropathy, but early mobilization in the ICU may improve mobility and muscle strength. 12,13 For painful polyneuropathy, gabapentin (Neurontin), pregabalin (Lyrica), or serotonin-norepinephrine reuptake inhibitors should be considered.
CRITICAL ILLNESS MYOPATHY
Critical illness (or ICU-acquired) myopathy also presents as muscle weakness and difficulty weaning from mechanical ventilation. The pathophysiology of critical illness myopathy is believed to be similar to that of critical illness polyneuropathy.10,11 Electromyography and nerve conduction velocity studies can assist with distinguishing between these two clinically similar diseases. Muscle biopsy is considered the diagnostic standard for critical illness myopathy.12 Unlike critical illness polyneuropathy, critical illness myopathy may improve with exercise. Early mobilization and physical therapy have the greatest evidence of benefit.10,11
MALNUTRITION
Early nutritional support has become the standard of care in the ICU and other inpatient settings and is associated with decreased rates of infection, length of hospitalization, and risk of readmission.14,15 Pre- and postadmission weight, appetite, and caloric intake should be assessed during post-ICU follow-up.
INSOMNIA
Among patients treated in the ICU, 10% to 61% report difficulty with sleep six months after discharge.16 Risk factors for insomnia include coexisting mental illness and higher opioid exposure in the ICU. Post-ICU insomnia is associated with increased levels of pain and decreased physical function.17 Patients should be asked about sleep quality and insomnia during post-ICU follow-up. Those with difficulty sleeping should be treated with nonpharmacologic interventions, including sleep hygiene, cognitive behavior therapy, and relaxation techniques.18
SEXUAL DYSFUNCTION
Sexual dysfunction occurs in up to 44% of patients treated in the ICU.19 There are no studies comparing the prevalence of sexual dysfunction in patients treated in the ICU with the general population, and there are no recommendations to screen for sexual dysfunction during post-ICU follow-up.
Psychological health should be assessed when sexual dysfunction is diagnosed because of the strong correlation between sexual dysfunction and posttraumatic stress disorder (PTSD). Treatment of post-ICU sexual dysfunction targets the underlying cause. Typical pharmacologic therapies, such as phosphodiesterase inhibitors, have not been studied in the post-ICU population.
Psychological Considerations
COGNITIVE IMPAIRMENT
There is a high prevalence of cognitive impairment in the ICU population, and several studies have shown persistence in cognitive impairment after discharge in more than 20% of patients.20,21 Up to 2.5% of patients develop new and persistent cognitive impairment post-ICU, with increasing risk associated with a higher number of comorbidities and ICU admissions.20 Hypotension, hypoxia, hyperthermia, delirium, and vancomycin or quinolone use may also increase the risk of cognitive impairment.20,21
Although there is limited evidence for specific treatments of cognitive impairment in the post-ICU setting, patients should be screened for cognitive impairment and, when present, evaluated for underlying reversible causes, such as depression, nutritional deficiencies, and metabolic disorders.20–22 Exercise, mental activity, and optimizing cardiovascular risk factors may be beneficial.22
DEPRESSION
Depressed mood and other mental health concerns are common in patients after ICU admission. Among patients treated in the ICU, 30% have at least mild depression 12 months after discharge.23,24 One study showed that depressive symptoms in patients treated in the ICU are more often somatic (e.g., fatigue, decreased appetite), and those with severe depression are more likely to experience concurrent PTSD.5 Depression has been associated with increased rates of mortality up to two years after ICU discharge.23
Patients should be screened for depression using a validated screening tool, such as the two- or nine-question Patient Health Questionnaire (available at https://www.aafp.org/afp/2018/1015/p508.html).5,24,25 If depression is diagnosed, appropriate behavior and/or pharmacologic therapy should be initiated.
ANXIETY
The prevalence of anxiety 12 months after ICU discharge ranges from 12% to 43%.25 More than 60% of patients with post-ICU anxiety disorder also have PTSD or depression.23 Patients should be screened for anxiety using a standardized screening tool, such as the General Anxiety Disorder-7 (available at https://www.mdcalc.com/gad-7-general-anxiety-disorder-7).5,24,25 If anxiety is diagnosed, appropriate behavior and/or pharmacologic therapy should be initiated.
POSTTRAUMATIC STRESS DISORDER
Among patients treated in the ICU, 7% to greater than 20% experience PTSD 12 months after discharge.5,23 Patients should be screened for PTSD using a standardized tool, such as the Primary Care PTSD Screen (available at https://www.ptsd.va.gov/professional/assessment/screens/pc-ptsd.asp).5,24,25 Review of an ICU diary (i.e., account of a patient's daily experiences while in the ICU) during the convalescence period has been shown to reduce the risk of PTSD.26
Because 18% to 31% of partners and family members of patients treated in the ICU also have PTSD symptoms six months after discharge, family physicians should consider screening them for PTSD, anxiety, and depression.27,28 Female sex, preexisting mental health disorders, personal history of recent serious illness, and being a partner or family member of a previously healthy patient are associated with higher rates of PTSD in this population.27,28
Medications
Reconciliation of pre- and post-ICU medications is vital for avoiding complications after discharge. Unintentional discontinuation of medications that treat chronic conditions is common on admission to the ICU.29 Family physicians should determine the indication, appropriateness, and duration for new medications prescribed at discharge. In one study, there were differences between medications prescribed at ICU discharge and medications documented in the outpatient setting in more than 40% of cases.30 Older patients may be prescribed potentially inappropriate medications (e.g., opioids, anticholinergics) in the ICU, and these medications are often continued at discharge.31 Medications for chronic conditions that were discontinued at discharge should be assessed and reinitiated only if appropriate.
Care Planning
Compared with the general population, patients treated in the ICU have increased rates of mortality up to 10 years after discharge.1 During post-ICU follow-up, family physicians should address patients' health care goals and end-of-life wishes and identify proxy health care decision-makers. Patient preferences regarding code status, artificial feeding, mechanical ventilation, and future ICU admission should be discussed. Tools such as the Serious Illness Conversation Guide (available at https://www.aafp.org/afp/2019/0301/p281.html) can help facilitate these conversations.
COVID-19 Considerations
Patients admitted to the ICU with COVID-19 are at risk of the typical post-ICU complications as well as additional complications, such as thrombotic and vascular issues.32 Management of COVID-19 after ICU treatment is evolving. A recent observational study showed that although the toll of COVID-19 extends well beyond hospitalization, one out of five patients hospitalized with COVID-19 have no primary care follow-up visit within 60 days of discharge.33
FOLLOW-UP AFTER HOSPITAL DISCHARGE
The Centers for Disease Control and Prevention recommends discontinuation of transmission precautions in those with initial mild to moderate illness when at least 10 days have passed since the onset of symptoms, the patient is afebrile without the use of antipyretics for at least 24 hours, and symptoms have improved.35 For patients with severe to critical illness or patients who are severely immunocompromised, transmission precautions should be continued for up to 20 days, and consultation with infection control experts should be considered. Because the Centers for Disease Control and Prevention no longer recommends a test-based strategy, patients discharged home must adhere to self-isolation and self-monitoring recommendations if transmission-based precautions remain in effect. Medications for chronic conditions should be continued with some exceptions (Table 2).34–36
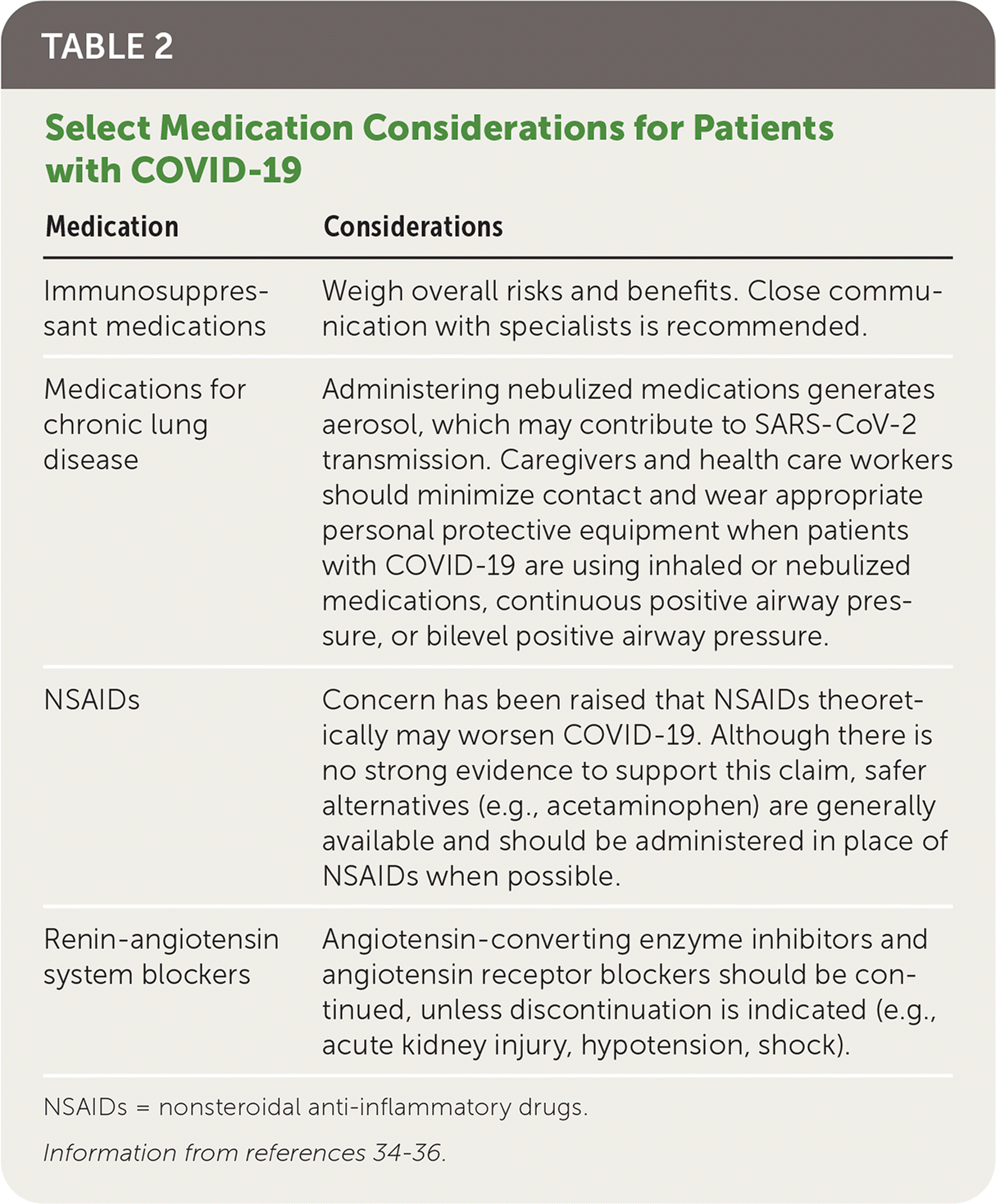
| Medication | Considerations |
|---|---|
| Immunosuppressant medications | Weigh overall risks and benefits. Close communication with specialists is recommended. |
| Medications for chronic lung disease | Administering nebulized medications generates aerosol, which may contribute to SARS-CoV-2 transmission. Caregivers and health care workers should minimize contact and wear appropriate personal protective equipment when patients with COVID-19 are using inhaled or nebulized medications, continuous positive airway pressure, or bilevel positive airway pressure. |
| NSAIDs | Concern has been raised that NSAIDs theoretically may worsen COVID-19. Although there is no strong evidence to support this claim, safer alternatives (e.g., acetaminophen) are generally available and should be administered in place of NSAIDs when possible. |
| Renin-angiotensin system blockers | Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers should be continued, unless discontinuation is indicated (e.g., acute kidney injury, hypotension, shock). |
REHABILITATION
Exercise and self-help rehabilitation programs improve muscle function and cardiopulmonary function after critical illness.7,8 With the emergence of telemedicine to allow for social distancing, telerehabilitation is a viable alternative for patients in isolation. Studies of patients with heart failure or stroke have shown that telerehabilitation is not inferior to traditional physical therapy.37,38 However, unsupervised exercise and competitive athletics should be avoided during the initial outpatient phase of recovery because up to 22% of patients hospitalized with COVID-19 have evidence of myocardial injury and/or myocarditis.39 Although evidence is limited, expert consensus suggests a gradual return to activity if recent cardiac biomarkers and electrocardiogram and echocardiogram findings were normal during admission and the patient has been asymptomatic for at least two weeks.39 Further cardiac testing may be considered in previously highly active individuals who did not undergo such testing during admission.39
TELEMEDICINE
Previous studies have shown similar health outcomes in those receiving care via telemedicine vs. traditional health care, and many patients report that they prefer virtual visits to in-person visits.40,41 Care provided via telemedicine was observed to be noninferior to traditional health care during other disease outbreaks, including severe acute respiratory syndrome, Middle East respiratory syndrome, and influenza.42 Advantages of telemedicine visits for patients who are recovering from COVID-19 or who have mild symptoms include reduced use of personal protective equipment and decreased exposure risk in communities, hospitals, and medical offices.
NURSING HOME CARE
Advance directives should be reviewed before transferring nursing home residents with suspected COVID-19 to the hospital for higher levels of care. Patients hospitalized with COVID-19 should be discharged to a nursing home or group care facility only if personal protective equipment is available and the patient can be isolated from other residents. Care facilities should implement screening protocols for staff and enhanced infection control policies.43
This article updates a previous article on this topic by Volk and Grassi.4
Data Sources: A literature search was conducted using PubMed, the Cochrane database, and Essential Evidence Plus. Search terms included post-ICU, post intensive care, and post critical care, individually and collectively. These terms were also paired with the following terms: mental illness, weakness, malnutrition, rehabilitation, cognition, COVID-19, and SARSCoV2. Search dates: May through December 2020.
