
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2021;104(1):58-62
Related Letter to the Editor: Additional Information on the Management of Infants Born to Mothers With HIV Infection
Patient information: See related handout on preventing HIV infection in babies, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
In the United States, approximately 5,000 women living with HIV infection give birth each year. HIV can be transmitted from a mother to her child at any time during pregnancy, labor and delivery, and breastfeeding. Because of effective preventive measures, the transmission rate from pregnant women to their children has declined significantly. Strategies to prevent mother-to-child transmission include maternal and infant antiretroviral therapy and formula-feeding instead of breastfeeding. All infants born to mothers with HIV infection should receive antiretroviral postexposure prophylaxis as soon as possible, ideally within six hours after delivery. The type of prophylaxis depends on whether the mother has achieved virologic suppression, defined by an HIV RNA load of less than 50 copies per mL, and if the infant is at high risk of vertical transmission of HIV. Risk factors for vertical transmission include maternal seroconversion during pregnancy or breastfeeding, high maternal plasma viral RNA load during pregnancy, and advanced maternal HIV disease.
In the United States, approximately 5,000 women living with HIV infection give birth each year.1 Since the initial Pediatric AIDS Clinical Trials Group Protocol 076 (PACTG 076) study was published in 1994, advances in the management of HIV infection have led to a dramatic decline in the incidence of perinatally infected infants.2,3 The annual rate of perinatal HIV transmission has decreased by more than 95% in the United States since the early 1990s.2,3 In 2017, only 73 infants were born with HIV infection in the United States. Five states (Florida, Texas, Georgia, Louisiana, and Maryland) accounted for 38% of infants born with HIV infection in the United States in 2016.4–6
Antenatal testing and treatment of pregnant women have reduced vertical transmission rates; however, opportunities remain to further decrease vertical transmission, and inadequate antenatal testing for HIV persists.7,8 The Centers for Disease Control and Prevention wants to eliminate perinatal HIV transmission in the United States, with a goal of reducing perinatal transmission to an incidence of less than one infection per 100,000 live births and a rate of less than 1% among HIV-exposed infants.8–10
Perinatal Transmission
Perinatal transmission of HIV can occur in pregnancy, labor and delivery, and breastfeeding, with the greatest risk during labor and delivery.11 Strategies to prevent mother-to-child transmission include giving antiretroviral therapy (ART) to mothers with HIV infection and their infants, scheduling cesarean deliveries for women with an HIV RNA load greater than 1,000 copies per mL or an unknown viral load at the time of delivery, and providing formula instead of breast milk to infants of mothers living with HIV. [corrected] Infants at highest risk of vertical transmission include those whose mothers have a viral load greater than 1,000 copies per mL within the four weeks before expected delivery, who received no ART or less than four weeks of ART by the time of delivery, who have advanced maternal HIV disease, or who acquired HIV infection during pregnancy or breastfeeding.11–13
Current Guidelines for Perinatal HIV Management
All pregnant women should be screened for HIV when establishing prenatal care and with each pregnancy.12 Those found to be HIV-positive should start ART.12 Repeat testing in the third trimester is recommended for women who are at increased risk of acquiring HIV infection (i.e., women who inject drugs, exchange sex for money or drugs, have sex partners with HIV infection, or have a sexually transmitted infection during pregnancy). Any woman who presents in labor without a documented HIV-negative test result should have expedited HIV testing.14 A positive test result should prompt intrapartum intravenous zidovudine (ZDV; Retrovir) for the mother and an antiretroviral regimen for the infant after delivery.14
A scheduled cesarean delivery at 38 weeks' gestation is recommended for pregnant patients with an HIV RNA load greater than 1,000 copies per mL or an unknown viral load at the time of delivery to minimize perinatal transmission of the virus.13,14 In the PACTG 076 study, antepartum ZDV, intrapartum ZDV, and six weeks of ZDV prophylaxis for the infant decreased perinatal transmission by 66%.3 Based on this study, continuous intravenous ZDV during labor was recommended for all pregnant women with HIV infection. However, a more recent study found that intrapartum ZDV did not affect the risk of perinatal HIV transmission among women with an HIV RNA viral load of less than 400 copies per mL at the time of delivery.15 Current recommendations state that intravenous ZDV should be administered only to women with an HIV RNA viral load greater than 1,000 copies per mL on polymerase chain reaction testing or if the viral load is unknown at the time of delivery.12,14,16
Management of the Infant After Delivery
All infants born to mothers with HIV infection should receive antiretroviral postexposure prophylaxis as soon as possible, ideally within six hours of delivery. The choice of prophylaxis depends on whether the mother has achieved virologic suppression and if the infant has risk factors known to increase perinatal transmission of the virus11–13 (Table 117). Previous guidelines recommended a six-week course of ZDV for all newborns exposed to maternal HIV infection.18 Newer evidence supports only four weeks of ZDV prophylaxis in infants if the mother has virologic suppression during pregnancy and near delivery and no concerns related to antiretroviral adherence.19,20
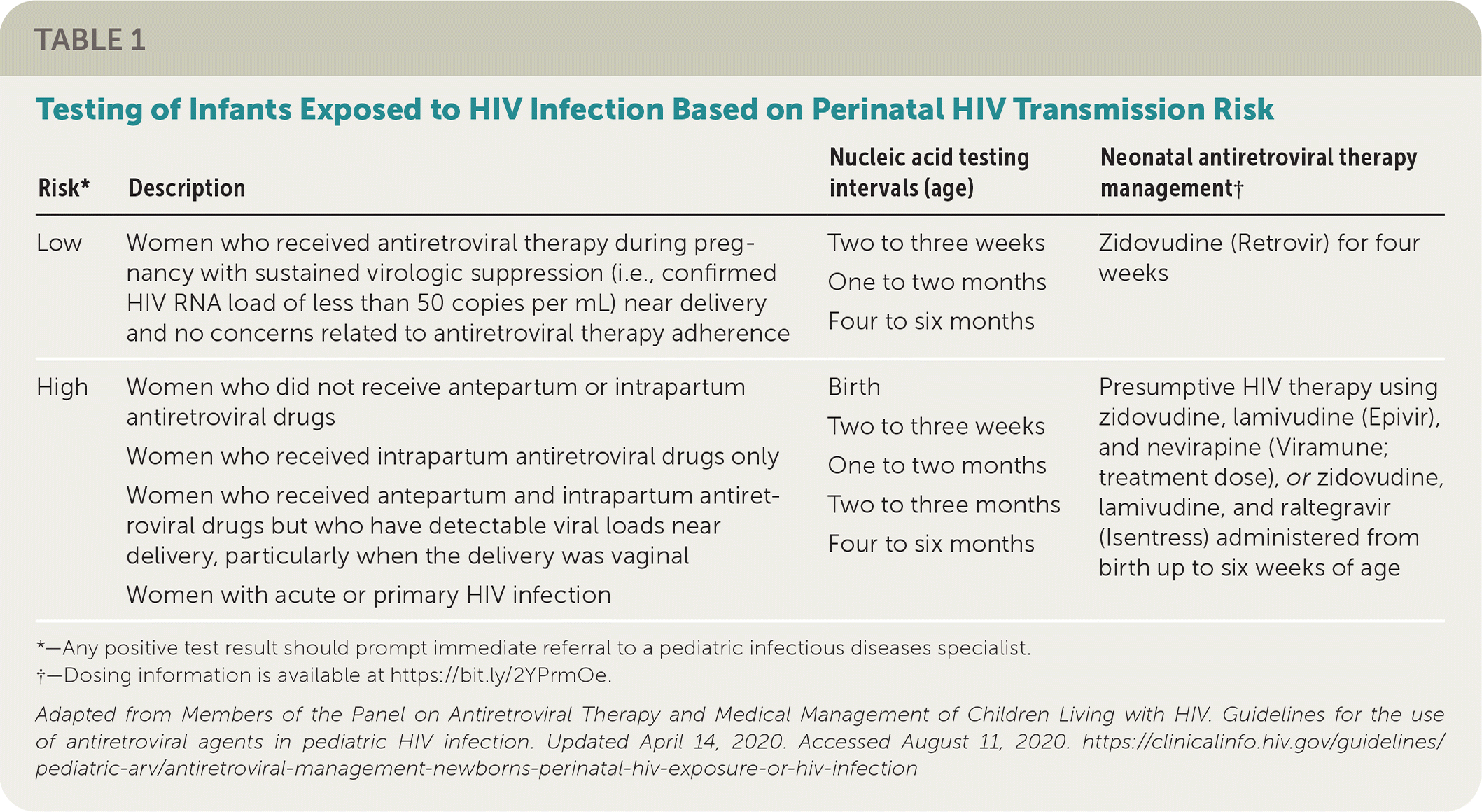
| Risk* | Description | Nucleic acid testing intervals (age) | Neonatal antiretroviral therapy management† |
|---|---|---|---|
| Low | Women who received antiretroviral therapy during pregnancy with sustained virologic suppression (i.e., confirmed HIV RNA load of less than 50 copies per mL) near delivery and no concerns related to antiretroviral therapy adherence | Two to three weeks One to two months Four to six months | Zidovudine (Retrovir) for four weeks |
| High | Women who did not receive antepartum or intrapartum antiretroviral drugs | Birth Two to three weeks One to two months Two to three months Four to six months | Presumptive HIV therapy using zidovudine, lamivudine (Epivir), and nevirapine (Viramune; treatment dose), or zidovudine, lamivudine, and raltegravir (Isentress) administered from birth up to six weeks of age |
| Women who received intrapartum antiretroviral drugs only | |||
| Women who received antepartum and intrapartum antiretroviral drugs but who have detectable viral loads near delivery, particularly when the delivery was vaginal | |||
| Women with acute or primary HIV infection |
For infants at high risk of perinatal acquisition of HIV infection, presumptive therapy for six weeks with a combination of three drugs (ZDV, lamivudine [Epivir], raltegravir [Isentress]) is recommended instead of ZDV monotherapy.12 This three-drug regimen serves as prophylaxis and is also the preliminary treatment for an infant with documented HIV infection.18,21–23
The use of antiretroviral drugs other than this regimen is not recommended in premature newborns (younger than 37 weeks' gestation) because of a lack of dosing and safety data.
Assessing HIV Infection Status in Exposed Infants
Infants born to mothers with HIV infection should have serial testing with an HIV nucleic acid test (NAT). Test selection depends on the age of the infant and the availability of the test. Virologic tests that detect HIV DNA or HIV RNA by polymerase chain reaction are used for diagnosis in infants. HIV antibody testing or combined HIV antigen or antibody assays should not be used in infants with perinatal or postnatal HIV exposure. These tests cannot distinguish between active infection and passive transfer of maternal HIV antibodies.18,24 Blood samples from the umbilical cord should not be used for diagnostic evaluation because of potential contamination with maternal blood.
The presumptive exclusion of HIV infection in nonbreastfed infants is based on two negative NATs (one test at four days of age and one test at four weeks of age), or one negative NAT at eight weeks of age, or one negative HIV antibody test at six months of age. The definitive exclusion of HIV infection in nonbreastfed infants is based on two or more negative NATs (one test at four weeks of age and one test at four months of age) or two negative antibody tests from separate specimens obtained at six months of age. Some experts perform antibody testing at 12 to 18 months to document clearance of maternal antibodies and to confirm the child's HIV seronegative status.18,24
Prophylaxis with trimethoprim/sulfamethoxazole should begin at four to six weeks of age if HIV infection cannot be presumptively excluded because of the risk of Pneumocystis jiroveci pneumonia in HIV-infected infants. Prophylaxis should be continued until HIV infection has been definitively or presumptively excluded. If HIV infection has been presumptively excluded, prophylaxis does not need to be initiated.18,24
Care of the HIV-Exposed Infant After Delivery
The newborn infant should be bathed and cleansed of maternal secretions at the time of birth. Antiretroviral prophylaxis should be initiated within six to 12 hours of delivery. A baseline complete blood count and differential should be obtained. The mother's prenatal care and exposure to infections such as syphilis, hepatitis B, hepatitis C, tuberculosis, and herpes simplex should be assessed and managed. Other infections to consider include Zika virus, toxoplasmosis, and cytomegalovirus. The mother should not be discharged from the hospital without antiretroviral medications for the baby.12 The infant should have an appointment with a family physician or pediatrician at two weeks of age to discuss medication adherence, possible adverse effects, and HIV diagnostic testing. If the infant's HIV test result is positive, the physician should contact a pediatric infectious diseases specialist for further management. Infants should be monitored closely for adverse effects resulting from ART, and hemoglobin and neutrophil counts should be measured four weeks after initiating ART.
Clinicians providing care to infants exposed to HIV infection should closely monitor them for growth and development and signs of acute HIV infection such as fever, rash, pneumonia, or opportunistic infections. All routinely recommended vaccines should be administered to infants exposed to HIV infection. The physician should counsel the family about methods to prevent postnatal HIV exposure. Women with HIV infection should not breastfeed their infants or donate to milk banks. Parents should also be educated about formula feeding, and infants should not receive solid foods before four to six months of age.24 Because of the potential for transmission of HIV through premastication of food, the Centers for Disease Control and Prevention recommends that physicians ask caregivers about the practice of premastication and counsel them not to premasticate food for their infants.18,24,25 For additional recommendations, see the American Academy of Pediatrics guideline on management of the infant exposed to HIV.26
This article updates a previous article on this topic by Krist and Crawford-Faucher.27
Data Sources: A PubMed search was completed in Clinical Queries using the key words perinatal HIV exposure, maternal to child transmission of HIV, and perinatal HIV prevention. The search included practice guidelines, meta-analyses, randomized controlled trials, and clinical trials. A PubMed search was also completed using the key words author name and publication year for relevant trials noted in guidelines. Also searched were Essential Evidence Plus, the Centers for Disease Control and Prevention, and the U.S. Department of Health and Human Services. Search dates: January 15, 2020; August 1, 2020; and December 18, 2020.
