Am Fam Physician. 2021;104(4):386-394
Related Letter to the Editor: Caution Before Antibiotic De-escalation Following Negative MRSA Nares Testing
Patient information: See related handout on preventing diabetic foot infections.
Author disclosure: No relevant financial affiliations.
Diabetes-related foot infections occur in approximately 40% of diabetes-related foot ulcers and cause significant morbidity. Clinicians should consider patient risk factors (e.g., presence of foot ulcers greater than 2 cm, uncontrolled diabetes mellitus, poor vascular perfusion, comorbid illness) when evaluating for a foot infection or osteomyelitis. Indicators of infection include erythema, induration, tenderness, warmth, and drainage. Superficial wound cultures should be avoided because of the high rate of contaminants. Deep cultures obtained through aseptic procedures (e.g., incision and drainage, debridement, bone culture) help guide treatment. Plain radiography is used for initial imaging if osteomyelitis is suspected; however, magnetic resonance imaging or computed tomography may help if radiography is inconclusive, the extent of infection is unknown, or if the infection orientation needs to be determined to help in surgical planning. Staphylococcus aureus and Streptococcus agalactiae are the most commonly isolated pathogens, although polymicrobial infections are common. Antibiotic therapy should cover commonly isolated organisms and reflect local resistance patterns, patient preference, and the severity of the foot infection. Mild and some moderate infections may be treated with oral antibiotics. Severe infections require intravenous antibiotics. Treatment duration is typically one to two weeks and is longer for slowly resolving infections or osteomyelitis. Severe or persistent infections may require surgery and specialized team-based wound care. Although widely recommended, there is little evidence on the effectiveness of primary prevention strategies. Systematic assessment, counseling, and comorbidity management are hallmarks of effective secondary prevention for diabetes-related foot infections.
Diabetes-related foot infections form in approximately 40% of foot ulcers in patients with diabetes mellitus.1 Infections can rapidly progress to cellulitis, abscess formation, osteomyelitis, and necrotizing fasciitis. In 2016, diabetes-related foot infections contributed to more than 130,000 lower-extremity amputations in the United States.2 The five-year mortality rate following amputation is approximately 50%, exceeding the mortality rate of many cancers.3
Pathophysiology
Patients with diabetes and vascular compromise, peripheral neuropathy, and impaired immune function are at high risk of developing foot infections. The risk increases with deformities (e.g., bunions, hammer toe, Charcot foot) that result in high compressive forces in certain areas of the foot.4 Peripheral neuropathy causes the loss of protective sensation for pain and temperature and increases the risk of foot trauma and ultimately foot ulceration. Approximately 50% of patients with neuropathy are asymptomatic, making recognition of a patient with an ulcer difficult.5 When the skin ulcerates, an infection can develop rapidly because of circulatory compromise and an impaired immune response. Infection can spread rapidly to surrounding tissues, initially causing cellulitis and later more severe complications such as osteomyelitis and necrotizing fasciitis.6
Microbiology
The most commonly isolated organisms from diabetes-related foot infections are the gram-positive bacteria Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus agalactiae (i.e., group B Streptococcus), and Enterococcus species. Wounds infected by methicillin-resistant S. aureus (MRSA) occur in approximately 15% of cases and are more serious considering the virulence of MRSA and the limited number of treatment options.7 Gram-negative bacteria are common and isolated in more than one-half of samples, particularly the Enterobacteriaceae group and Pseudomonas aeruginosa.8 Anaerobes are present in about one-third of cultures. Bacteroides fragilis, Prevotella, Porphyromonas, and Clostridium species are the most common.9 Approximately 50% to 80% of infections are polymicrobial, which complicates treatment.10
Diagnostic Evaluation
Prompt diagnosis of a diabetes-related foot infection decreases the risk of morbidity and mortality. Family physicians should consider patient risk factors (e.g., presence of foot ulcers greater than 2 cm, uncontrolled diabetes, poor vascular perfusion, comorbid illness) when assessing for infection. Findings suggestive of infection include erythema, induration, tenderness, warmth, and drainage. The probe-to-bone test is an office maneuver that is 87% sensitive and 83% specific for osteomyelitis.11 A probe-to-bone test result is positive if insertion of a sterile and blunt metal instrument is met with hard or gritty resistance. An erythrocyte sedimentation rate greater than 70 mm per hour is also suggestive of osteomyelitis.4,6 Other causes of inflammation (e.g., gout, rheumatoid arthritis, trauma) should be clinically ruled out.
Although an elevated white blood cell count can indicate a more severe infection, it is not often elevated with a diabetes-related foot infection. C-reactive protein and procalcitonin correlate better to soft tissue bacterial infections than erythrocyte sedimentation rate and white blood cell count.6 Routine superficial wound cultures should be avoided because of the high rate of contaminants; however, deep tissue cultures obtained using aseptic procedures (i.e., incision and drainage, debridement, and bone culture) help guide treatment.6,12 A negative MRSA nares culture reduces the likelihood that a diabetes-related foot infection is caused by MRSA. Studies have shown correlations with negative predictive values between 73% and 90%.13,14
Plain radiography should be the initial imaging test if osteomyelitis is suspected.6,15 Osteomyelitis can take weeks to appear on radiographs; therefore, magnetic resonance imaging (MRI) or computed tomography (CT) is warranted if a concern for osteomyelitis persists with normal radiography findings. MRI helps detect soft tissue involvement and identifies the spatial orientation of infection to guide surgical planning. CT is appropriate if MRI is contraindicated.15
Vascular assessment should be performed on presentation, and patients with nonpalpable pulses should be formally evaluated for arterial insufficiency.16 Approximately 10% to 40% of people with diabetes have peripheral arterial disease.17 An ankle-brachial index is a quick and affordable way to assess blood flow, but it can be inaccurate because of arterial calcification with diabetes.16,18 Transcutaneous oximetry or arterial duplex ultrasonography may improve the accuracy of the vascular assessment.17,19 For more urgent detection of arterial disease, magnetic resonance angiography with and without intravenous contrast media or a CT with intravenous contrast media is preferred. If a patient is unable to receive intravenous contrast media because of renal disease, duplex ultrasonography of the lower extremity or magnetic resonance angiography without contrast media are appropriate alternatives.20
GRADING SEVERITY
In 2019, the International Working Group on the Diabetic Foot published an update to the grading severity scale for diagnosing and classifying the extent of diabetes-related foot infections. This scale is the most validated scoring system to grade the severity of the infection and is summarized in Table 1.6 The scale scores a foot ulcer from 1 to 4 (1 = uninfected, 2 = mild infection, 3 = moderate infection, 4 = severe infection) and an “(O)” may follow scores 3 or 4 to indicate osteomyelitis.

| Clinical classification of infection, with definitions | IWGDF classification |
|---|---|
| No systemic or local symptoms or signs of infection Infected: At least two of these items are present: Local swelling or induration Erythema > 0.5 cm* around the wound Local tenderness or pain Local increased warmth Purulent discharge And no other cause of an inflammatory response of the skin (e.g., trauma, gout, acute Charcot neuro-osteoarthropathy, fracture, thrombosis, venous stasis) | 1 (uninfected) |
| Infection with no systemic manifestations (see below) involving: Only the skin or subcutaneous tissue (not any deeper tissues) and Any erythema present does not extend > 2 cm† around the wound | 2 (mild infection) |
| Infection with no systemic manifestations and involving: Erythema extending 2 cm* from the wound margin, and/or Tissue deeper than skin and subcutaneous tissues (e.g., tendon, muscle, joint, bone) | 3 (moderate infection) |
| Any foot infection with associated systemic manifestations (of the systemic inflammatory response syndrome [SIRS]), as manifested by 2 of the following: Temperature > 38°C or < 36°C Heart rate > 90 beats per minute Respiratory rate > 20 breaths per minute or PaCO2 < 4.3 kPa (32 mm Hg) White blood cell count > 12,000 per mm3, < 4,000 per mm3, or > 10% immature (band) forms | 4 (severe infection) |
| Infection involving bone (osteomyelitis) | Add “(O)” after 3 or 4‡ |
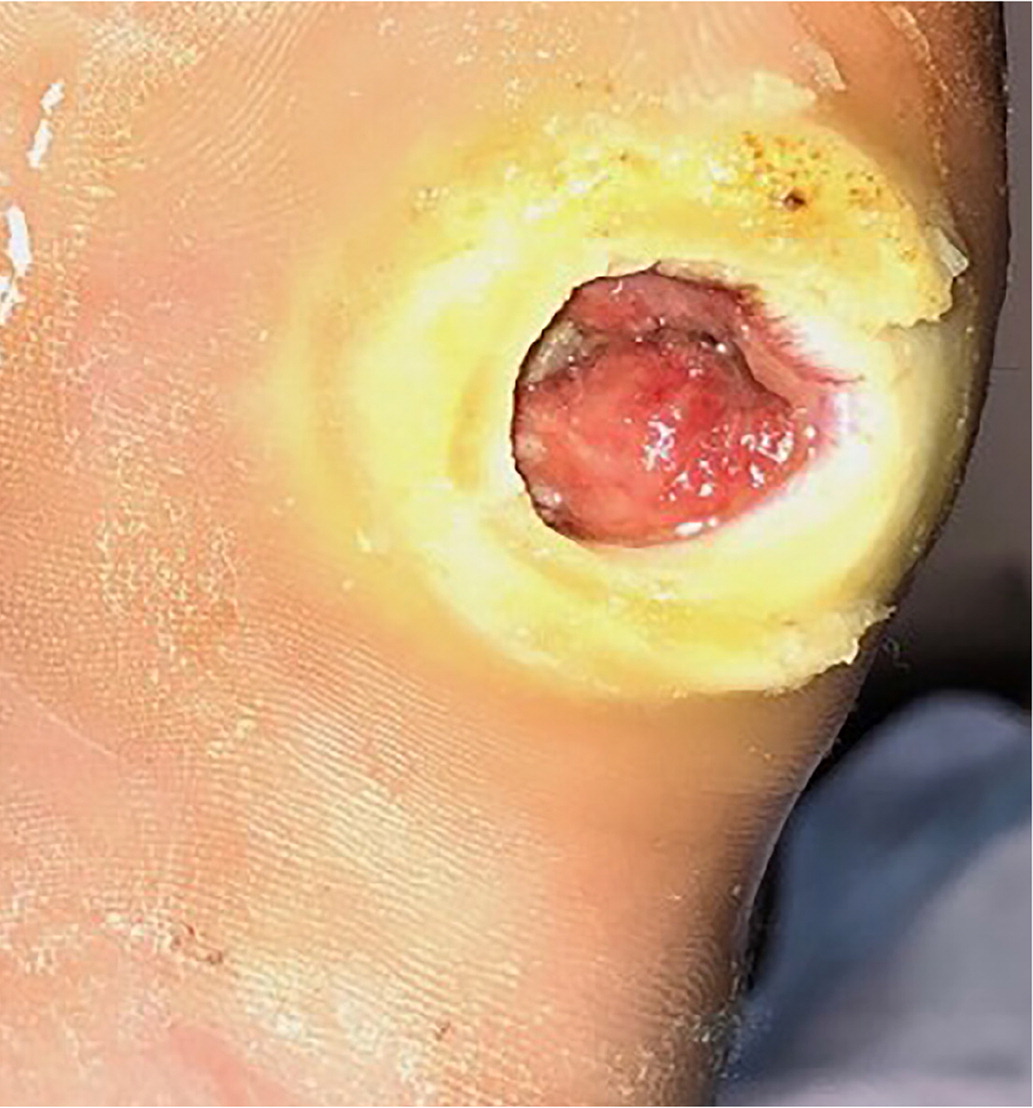
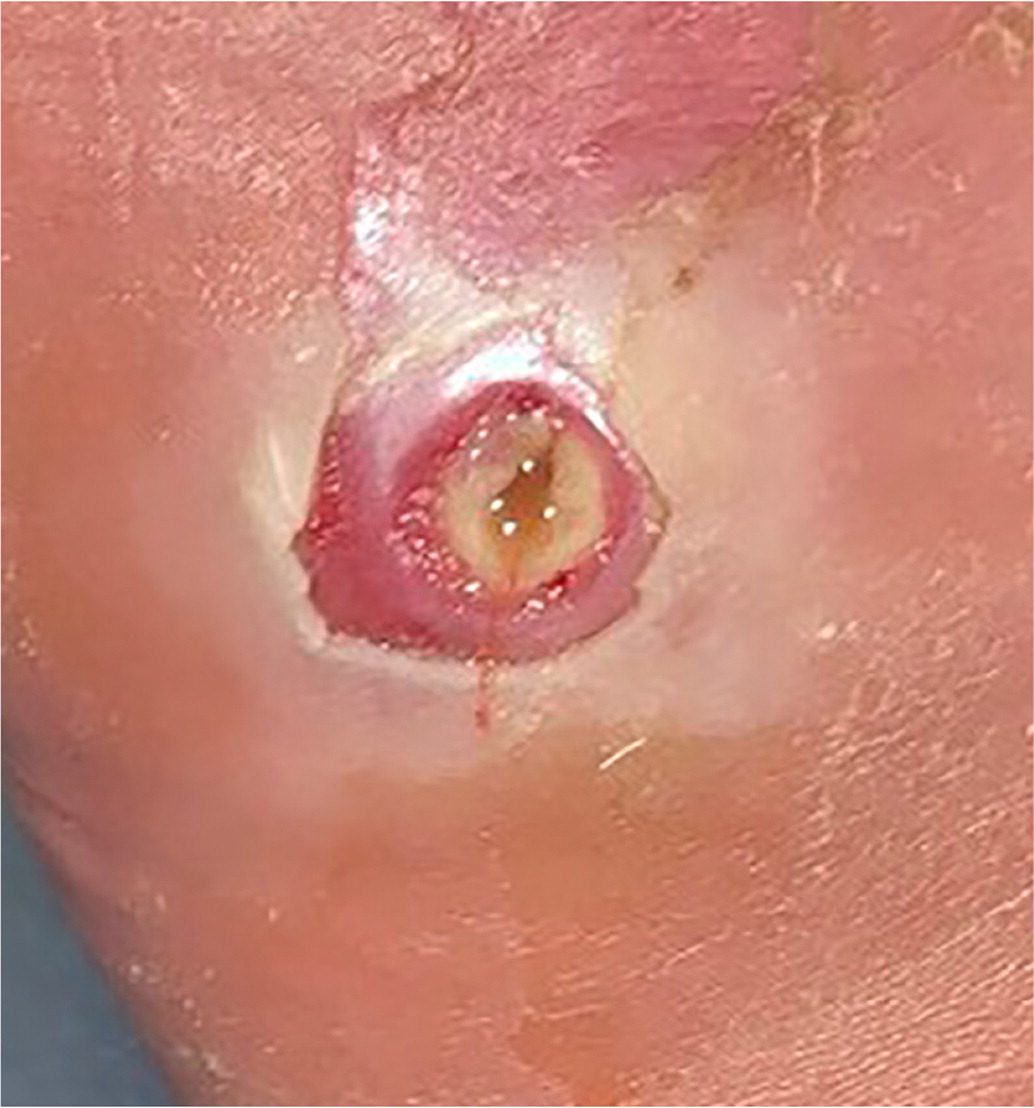
Erythema from a diabetes-related foot infection does not have to be contiguous to a foot ulcer in the updated classification scheme. 6 Scores of 3 (odds ratio [OR] = 1.7) or 4 (OR = 2.5) are associated with increased amputation rates.21 Other validated tools include the Site, Ischemia, Neuropathy, Bacterial Infection, and Depth scoring system and the Wound, Ischemia, foot Infection scale, which help predict outcomes and guide decisions for surgical interventions.18,22 The Perfusion, Extent, Depth, Infection, and Sensation score is a validated scale to predict amputation and mortality at six months and is available as an online calculator (https://www.mdcalc.com/pedis-score-diabetic-foot-ulcers).23,24 Figure 1 shows an uninfected ulcer, and Figure 2 shows an infected diabetes-related foot ulcer.
Treatment
ANTIBIOTIC THERAPY
Clinicians choosing antibiotics to treat patients with a diabetes-related foot infection should consider local antimicrobial sensitivities, the severity of infection, patient factors (e.g., drug-drug interactions, drug-disease interactions, renal dysfunction, drug allergies), previous antibiotic response, and patient preference. It is unclear if any one antibiotic is superior for resolving an infection or safer than other antibiotics.25 Empiric antibiotic coverage for a mild infection should include S. aureus and S. agalactiae.6 Guidelines also recommend empiric coverage for MRSA. A negative MRSA nares culture may help clinicians de-escalate MRSA-specific coverage considering the high negative predictive value of this test.6,13,14 Empiric antibiotic coverage for gram-negative rods (including P. aeruginosa) and anaerobes is reserved for moderate or severe infections, recurrent infections, or infections with severe limb ischemia.6 Antibiotics used to treat diabetes-related foot infections are summarized in Table 2.26–28
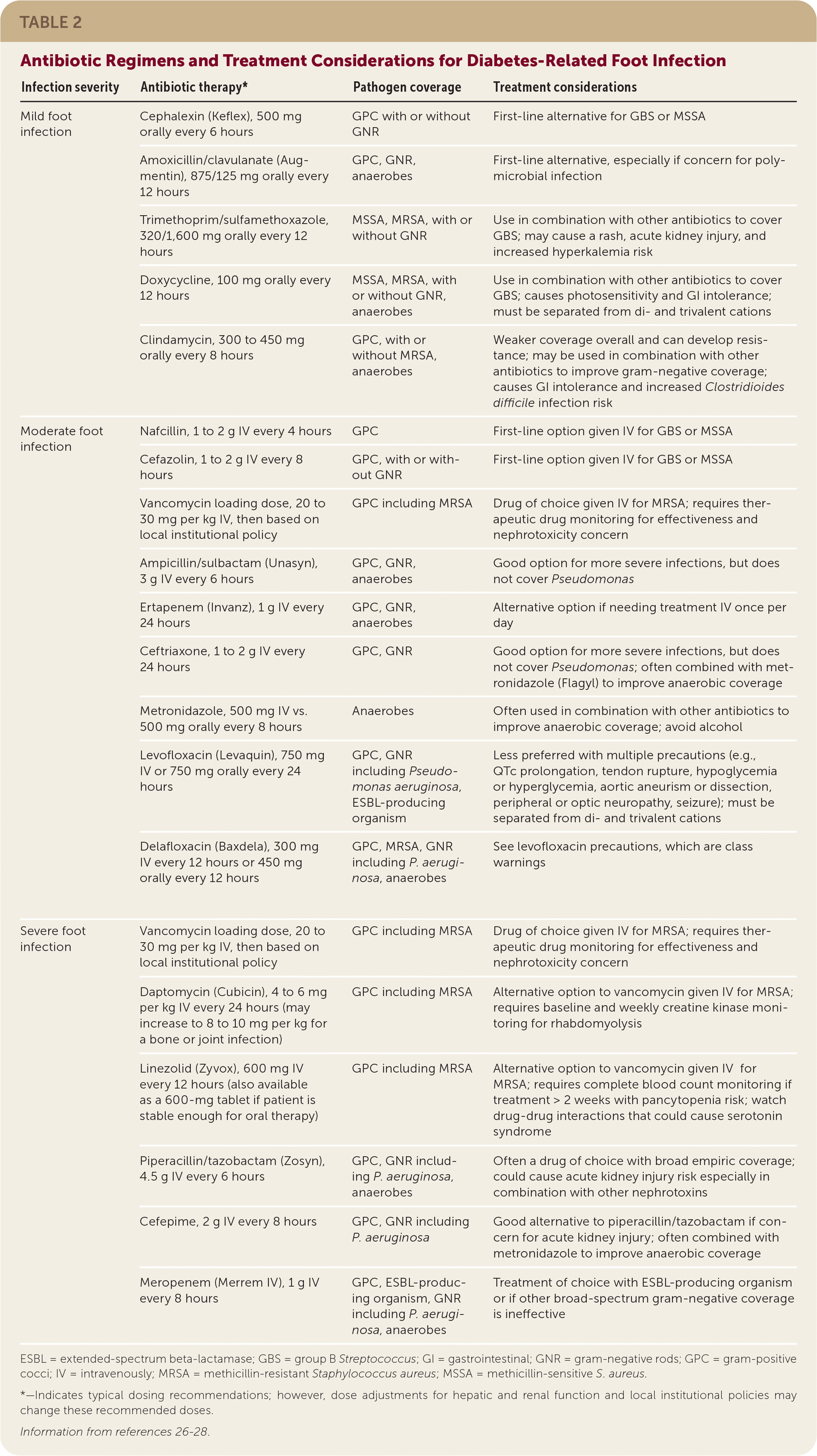
| Infection severity | Antibiotic therapy* | Pathogen coverage | Treatment considerations | |
|---|---|---|---|---|
| Mild foot infection | Cephalexin (Keflex), 500 mg orally every 6 hours | GPC with or without GNR | First-line alternative for GBS or MSSA | |
| Amoxicillin/clavulanate (Augmentin), 875/125 mg orally every 12 hours | GPC, GNR, anaerobes | First-line alternative, especially if concern for polymicrobial infection | ||
| Trimethoprim/sulfamethoxazole, 320/1,600 mg orally every 12 hours | MSSA, MRSA, with or without GNR | Use in combination with other antibiotics to cover GBS; may cause a rash, acute kidney injury, and increased hyperkalemia risk | ||
| Doxycycline, 100 mg orally every 12 hours | MSSA, MRSA, with or without GNR, anaerobes | Use in combination with other antibiotics to cover GBS; causes photosensitivity and GI intolerance; must be separated from di- and trivalent cations | ||
| Clindamycin, 300 to 450 mg orally every 8 hours | GPC, with or without MRSA, anaerobes | Weaker coverage overall and can develop resistance; may be used in combination with other antibiotics to improve gram-negative coverage; causes GI intolerance and increased Clostridioides difficile infection risk | ||
| Moderate foot infection | Nafcillin, 1 to 2 g IV every 4 hours | GPC | First-line option given IV for GBS or MSSA | |
| Cefazolin, 1 to 2 g IV every 8 hours | GPC, with or without GNR | First-line option given IV for GBS or MSSA | ||
| Vancomycin loading dose, 20 to 30 mg per kg IV, then based on local institutional policy | GPC including MRSA | Drug of choice given IV for MRSA; requires therapeutic drug monitoring for effectiveness and nephrotoxicity concern | ||
| Ampicillin/sulbactam (Unasyn), 3 g IV every 6 hours | GPC, GNR, anaerobes | Good option for more severe infections, but does not cover Pseudomonas | ||
| Ertapenem (Invanz), 1 g IV every 24 hours | GPC, GNR, anaerobes | Alternative option if needing treatment IV once per day | ||
| Ceftriaxone, 1 to 2 g IV every 24 hours | GPC, GNR | Good option for more severe infections, but does not cover Pseudomonas; often combined with metronidazole (Flagyl) to improve anaerobic coverage | ||
| Metronidazole, 500 mg IV vs. 500 mg orally every 8 hours | Anaerobes | Often used in combination with other antibiotics to improve anaerobic coverage; avoid alcohol | ||
| Levofloxacin (Levaquin), 750 mg IV or 750 mg orally every 24 hours | GPC, GNR including Pseudomonas aeruginosa, ESBL-producing organism | Less preferred with multiple precautions (e.g., QTc prolongation, tendon rupture, hypoglycemia or hyperglycemia, aortic aneurism or dissection, peripheral or optic neuropathy, seizure); must be separated from di- and trivalent cations | ||
| Delafloxacin (Baxdela), 300 mg IV every 12 hours or 450 mg orally every 12 hours | GPC, MRSA, GNR including P. aeruginosa, anaerobes | See levofloxacin precautions, which are class warnings | ||
| Severe foot infection | Vancomycin loading dose, 20 to 30 mg per kg IV, then based on local institutional policy | GPC including MRSA | Drug of choice given IV for MRSA; requires therapeutic drug monitoring for effectiveness and nephrotoxicity concern | |
| Daptomycin (Cubicin), 4 to 6 mg per kg IV every 24 hours (may increase to 8 to 10 mg per kg for a bone or joint infection) | GPC including MRSA | Alternative option to vancomycin given IV for MRSA; requires baseline and weekly creatine kinase monitoring for rhabdomyolysis | ||
| Linezolid (Zyvox), 600 mg IV every 12 hours (also available as a 600-mg tablet if patient is stable enough for oral therapy) | GPC including MRSA | Alternative option to vancomycin given IV for MRSA; requires complete blood count monitoring if treatment > 2 weeks with pancytopenia risk; watch drug-drug interactions that could cause serotonin syndrome | ||
| Piperacillin/tazobactam (Zosyn), 4.5 g IV every 6 hours | GPC, GNR including P. aeruginosa, anaerobes | Often a drug of choice with broad empiric coverage; could cause acute kidney injury risk especially in combination with other nephrotoxins | ||
| Cefepime, 2 g IV every 8 hours | GPC, GNR including P. aeruginosa | Good alternative to piperacillin/tazobactam if concern for acute kidney injury; often combined with metronidazole to improve anaerobic coverage | ||
| Meropenem (Merrem IV), 1 g IV every 8 hours | GPC, ESBL-producing organism, GNR including P. aeruginosa, anaerobes | Treatment of choice with ESBL-producing organism or if other broad-spectrum gram-negative coverage is ineffective | ||
Oral antibiotics are appropriate for individuals with mild infection and some moderate infections, whereas intravenous antibiotics are always needed initially for a severe infection, including individuals with osteomyelitis.4,6 After the infection improves on intravenous antibiotics, it is reasonable to switch to an oral antibiotic. Oral antibiotics can also be used for osteomyelitis after five to seven days of intravenous coverage if the oral regimen has a high bioavailability.6
The optimal duration of antibiotic therapy for a diabetes-related foot infection depends on how quickly the infection improves, the severity of infection, and patient factors (e.g., peripheral vascular disease, antibiotic adherence, adverse antibiotic effects).29 Most patients should receive one to two weeks of antibiotics; however, treatment could be extended to three to four weeks for slowly resolving infections.4,6 Antibiotics may be needed for only a few days if osteomyelitis is surgically treated with amputation. Guidelines have recommended four to six weeks of antibiotics if osteomyelitis is not treated surgically, but recent evidence suggests three weeks of therapy may be similar to six weeks.4,6,30
Topical antibiotics are commonly applied to dressings for the prevention and treatment of mild diabetes-related foot infections. Resolution of a foot infection may be faster with this approach, although the data supporting topical antibiotics is weak and based on poorly designed trials.31
SURGICAL TREATMENT
Surgical treatment plays a significant role in the management of diabetes-related foot infection. Tissue and bone cultures obtained during surgical interventions help guide antibiotic selection. Many patients need sharp surgical debridement by a wound care clinician or surgeon to remove necrotic tissue or calluses and aid in the formation of granulation tissue capable of re-epithelialization.6 Shared decision-making with patients is important because surgical procedures range from bedside debridement to major amputation. Amputations are devastating psychologically, and many patients fear amputation more than death.32
Surgical intervention is needed for gangrene, necrotizing fasciitis, or significant abscess formation. Although surgical resection of osteomyelitis was traditionally the standard of care, emerging evidence suggests most infections respond well to antibiotic therapy alone.6
In patients with a diabetes-related foot infection and ischemia, vascular interventions should be considered to improve a patient's treatment response and lower the risk of recurrence.17 The Wound, Ischemia, foot Infection score predicts clinical outcomes and guides interventions in patients with more advanced disease.33,34 The Wound, Ischemia, foot Infection scoring system factors in the International Working Group on the Diabetic Foot infection grade, objective measures to determine the extent of ischemia, and the anticipated likelihood of wound healing. These factors combine to stage wounds from 0 to 3 with higher scores requiring more invasive surgical management, including amputation.18
OTHER THERAPIES
Wound therapy in a patient with a diabetes-related foot infection is complex and often requires team-based care. Comprehensive wound care may include debridement, application of moist dressings, and the use of off-loading orthotics to reduce pressure on a wound.6 A moist dressing is preferred to aid in healing and help with infection control. It is unknown if any specific dressing is more effective because of a lack of head-to-head trials.35 Redistribution of pressure off the plantar surface is important because this is the main cause of foot ulcers and, if not addressed, may inhibit ulcer healing. Strategies to help with off-loading pressure include changes to a patient's shoes, specialized boots, or orthotic walkers.36
Studies of adjunctive treatments (e.g., hyperbaric oxygen therapy, maggot debridement therapy, granulocyte colony-stimulating factors, topical oxygen therapy, laser therapy) for healing diabetes-related foot ulcers have mixed results. Of these alternative treatments, hyperbaric oxygen therapy has the best data, with evidence showing that it lowers the risk of major amputations and improves wound healing; however, evidence does not support reductions in minor amputations or mortality.37 Maggot debridement therapy has good data, with evidence for shortening ulcer healing time and reducing the rate of amputations.38 Granulocyte colony-stimulating factors have not been shown to help resolve an infection or foot ulcer significantly, but they may decrease the risk of surgical interventions and amputations.39 Promising evidence exists for topical oxygen therapy and laser therapy for improving diabetes-related foot ulcer healing; however, more evidence is needed on patient-oriented outcomes before widespread adoption of either intervention.40,41
Prevention
Little evidence exists for primary prevention strategies of diabetes-related foot ulcers or infections despite widespread support for these interventions.42 Guidelines strongly support systematic assessment, foot care counseling, and comorbidity management for primary prevention because these strategies are useful in secondary prevention, and complications from a diabetes-related foot infection are significant.6,43 Recognition of a patient with neuropathy is critical considering the high rate of patients who are asymptomatic. Conducting a foot examination may take only three minutes and can be organized into three parts (patient history, physical examination, patient education).5 Team-based care for primary prevention may include nurses, pharmacists, podiatrists, and other clinicians.
Secondary prevention of diabetes-related foot ulcers and infections starts with frequent, systematic assessments recommended by guidelines such as the American Diabetes Association's Standards of Medical Care. These guidelines highlight the importance of a comprehensive foot examination at least annually, and for every diabetes care visit for individuals at high risk of an infection (e.g., poor circulation, history of amputation, severe neuropathy).43 All patients with diabetes should receive counseling on foot care and how to choose appropriate footwear. Using therapeutic footwear is often unnecessary; however, it should be considered in high-risk patients (e.g., severe neuropathy, foot deformities, ulcers, poor circulation, history of amputation).43
Other preventive techniques include improving glucose control, smoking cessation, daily foot inspection, debridement of calluses, and monthly physician foot checks for patients with end-stage renal disease requiring dialysis.42–45 Interventions to prevent an ulcer or diabetes-related foot infections are summarized in Table 3.5,42–45
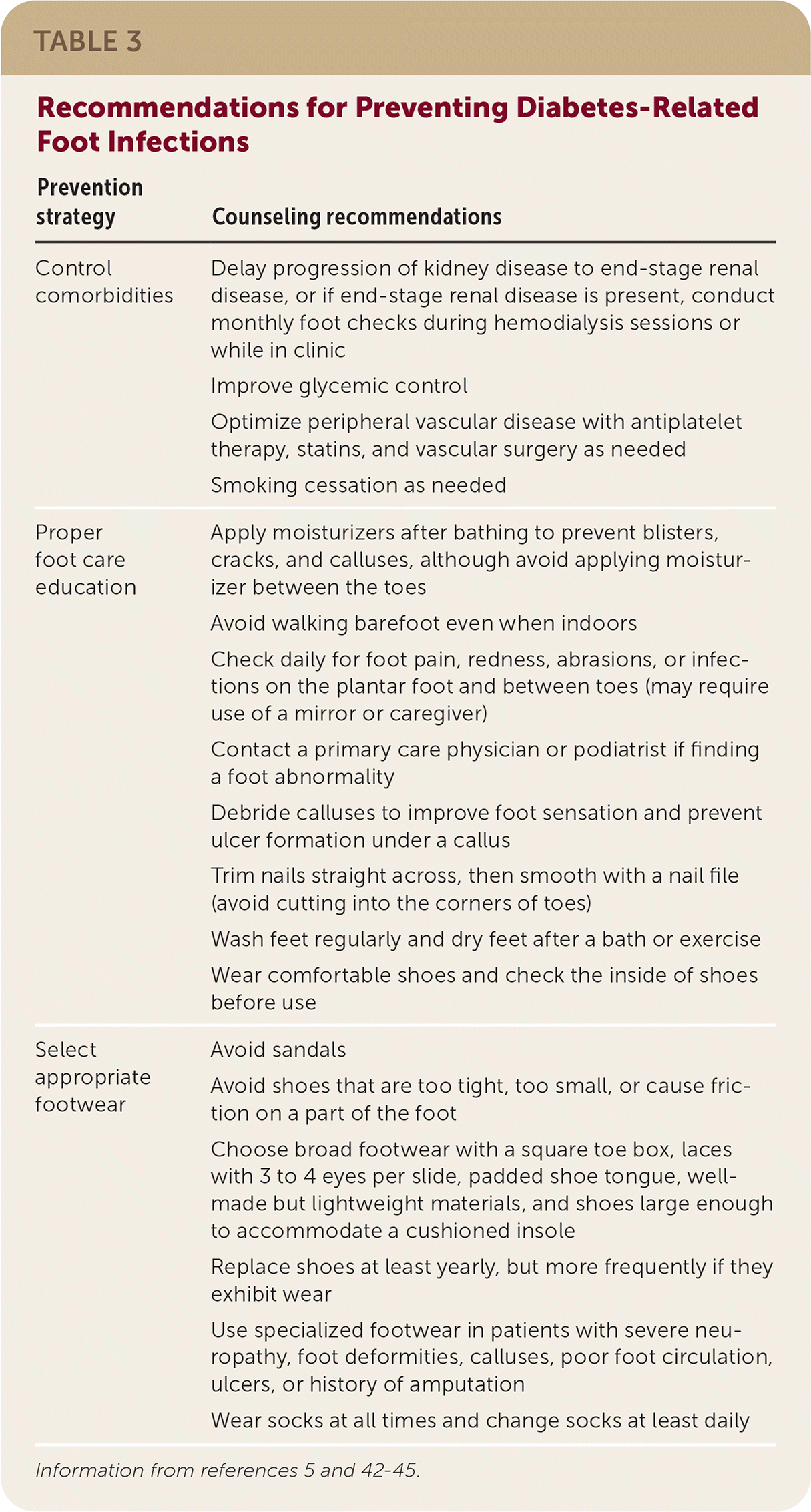
| Prevention strategy | Counseling recommendations |
|---|---|
| Control comorbidities | Delay progression of kidney disease to end-stage renal disease, or if end-stage renal disease is present, conduct monthly foot checks during hemodialysis sessions or while in clinic |
| Improve glycemic control | |
| Optimize peripheral vascular disease with antiplatelet therapy, statins, and vascular surgery as needed | |
| Smoking cessation as needed | |
| Proper foot care education | Apply moisturizers after bathing to prevent blisters, cracks, and calluses, although avoid applying moisturizer between the toes |
| Avoid walking barefoot even when indoors | |
| Check daily for foot pain, redness, abrasions, or infections on the plantar foot and between toes (may require use of a mirror or caregiver) | |
| Contact a primary care physician or podiatrist if finding a foot abnormality | |
| Debride calluses to improve foot sensation and prevent ulcer formation under a callus | |
| Trim nails straight across, then smooth with a nail file (avoid cutting into the corners of toes) | |
| Wash feet regularly and dry feet after a bath or exercise | |
| Wear comfortable shoes and check the inside of shoes before use | |
| Select appropriate footwear | Avoid sandals |
| Avoid shoes that are too tight, too small, or cause friction on a part of the foot | |
| Choose broad footwear with a square toe box, laces with 3 to 4 eyes per slide, padded shoe tongue, well-made but lightweight materials, and shoes large enough to accommodate a cushioned insole | |
| Replace shoes at least yearly, but more frequently if they exhibit wear | |
| Use specialized footwear in patients with severe neuropathy, foot deformities, calluses, poor foot circulation, ulcers, or history of amputation | |
| Wear socks at all times and change socks at least daily |
This article updates previous articles on this topic by Gemechu, et al.,27 and Bader.26
Data Sources: A PubMed search was completed in Clinical Queries using the key terms diabetic foot ulcers, infections, antibiotics, statistics, pharmacological, and prevention. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were Access Medicine, the Cochrane Library, Lexicomp, the National Guideline Clearinghouse database, and UpToDate. Search dates: October 27, 2020 to November 4, 2020, and April 26, 2021.