
Am Fam Physician. 2022;105(3):309-310
Author disclosure: No relevant financial relationships.
Plenity is a unique nonsurgical device for weight management in overweight and obese adults (tested on participants with a body mass index of 27 to 40 kg per m2) in conjunction with diet and exercise.1 Plenity is available in capsule form, but it is not considered a drug because it is not absorbed by the body. Rather, the hydro-gel capsule releases gel particles containing cellulose and citric acid that absorb water in the stomach and small intestine, expanding significantly in size. This action works to create increased bulk, thus signaling satiety. The particles break down in the colon and are excreted in the stool without being absorbed. For these reasons, Plenity is considered a medical device rather than a drug, leading to a different threshold for approval. It is currently approved by the U.S. Food and Drug Administration.
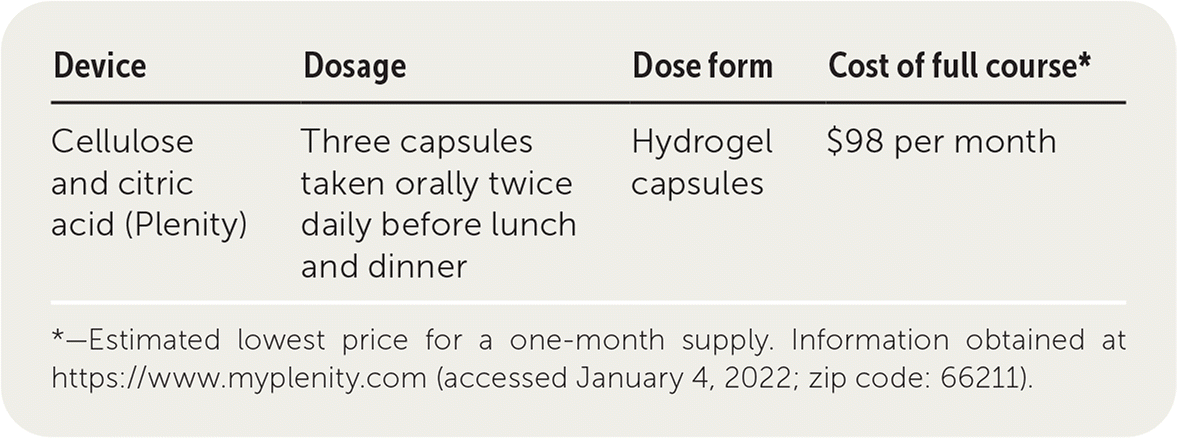
| Device | Dosage | Dose form | Cost of full course* |
|---|---|---|---|
| Cellulose and citric acid (Plenity) | Three capsules taken orally twice daily before lunch and dinner | Hydrogel capsules | $98 per month |
Safety
In a single clinical trial of 436 patients, there were no differences in rates of adverse effects between those taking Plenity (n = 223) and those taking placebo (n = 213). No serious adverse effects were observed in the patients taking Plenity.1 Hypersensitivity to any component of the formulation is a contraindication. Plenity may interfere with medication absorption and should not be taken at the same time as other medications. Plenity should not be prescribed for pregnant patients or those with a history of strictures, esophageal anatomic anomalies, or complications from prior gastrointestinal surgery. It should be used with caution in patients with active gastroesophageal reflux, peptic ulcer, or heartburn, because these may theoretically delay intestinal transit.2
Tolerability
About 40% of patients taking Plenity will experience abdominal distention, abdominal pain, changes in bowel movements, and bloating. These rates are similar to those reported with a placebo. In a premarketing study of 436 people, dropout rates from adverse effects (4%) were similar in the treatment and placebo groups.1
Effectiveness
Data are limited about the effectiveness of Plenity. It has been studied in a single published trial of 436 patients with an average BMI of 34 kg per m2 who were randomized to receive treatment or placebo in addition to calorie restriction. Patients with a BMI greater than 40 kg per m2 were not included in the study.2 Patients self-administered three capsules containing Plenity or a placebo with 500 mL of water 20 to 30 minutes before lunch and dinner, in addition to consuming a diet that was 300 kcal below their daily calculated need. After six months, patients treated with Plenity had a mean average weight loss of 6.4% compared with 4.4% in those receiving placebo. Significantly more patients treated with Plenity (59% vs. 42% with placebo) achieved a weight loss of at least 5%, and 27% (vs. 15% with placebo) lost at least 10% of their body weight. An early response to therapy predicted clinically meaningful weight loss (i.e., 5% or more) at 24 weeks.2
There was no significant change in cardiovascular risk markers over six months of treatment compared with placebo. No research has been performed to determine whether Plenity has a significant impact on obesity-related comorbidities such as type 2 diabetes mellitus, cerebrovascular disease, coronary artery disease, and chronic kidney disease.
Price
Plenity costs $98 for a one-month supply. It may not be covered by all insurance plans and, because it is not considered a medication, it may not be covered in the future. In comparison, phentermine costs about $15 per month, and generic topiramate costs $20 per month.
Simplicity
Patients should take three capsules twice per day with 500 mL of water 20 minutes before lunch and dinner. Plenity is not dispensed in pharmacies. Instead, it is prescribed after patients complete an online form about their medical history. No in-person examination is required.
Bottom Line
Plenity, in combination with moderate calorie restriction, may be an effective option for adults wishing to lose weight, at least in the short term. However, as a medical device, Plenity has not been held to the same standard of testing as would be expected with medications.
