This is an updated version of the article that appeared in print.
Am Fam Physician. 2022;106(3):251-259
Related editorial: Contraception Recommendations: Updates for the Busy Clinician
Related letter to the editor: Physicians Need Education About Fertility Awareness–Based Methods
Published online May 27, 2022.
Author disclosure: No relevant financial relationships.
Primary care clinicians are uniquely situated to reduce unintended pregnancy in the context of a patient's medical comorbidities, social circumstance, and gender identity. New evidence regarding contraception use has emerged in recent years. The copper intrauterine device is the most effective option for emergency contraception, with similar effectiveness found for the levonorgestrel-releasing intrauterine system, 52 mg, and both offer extended future contraception. Ulipristal given within 120 hours after unprotected intercourse is the most effective oral emergency contraceptive. Oral levonorgestrel, 1.5 mg, is slightly less effective than ulipristal, and is less effective in patients with a body mass index of more than 30 kg per m2 and if administered after 72 hours. The Yuzpe method, which uses a combination of oral contraceptives, is less effective than ulipristal or oral levonorgestrel, 1.5 mg, and has high risk of nausea and vomiting. Contraception methods based on fertility awareness are safe and have similar effectiveness as condom use and the withdrawal method. Patients who have migraine with aura have a higher risk of ischemic stroke, and combined oral contraceptives appear to increase this risk. Therefore, the Centers for Disease Control and Prevention recommends avoiding their use in these patients. Studies support the extended use of the levonorgestrel-releasing intrauterine system, 52 mg, for eight years, the copper intrauterine device for 12 years, and the etonogestrel subdermal contraceptive implant for five years. One levonorgestrel-releasing intrauterine device, 52 mg, (Mirena) was recently approved by the U.S. Food and Drug Administration (FDA) for eight years of use to prevent pregnancy. However, the intervals for the copper intrauterine device and the etonogestrel subdermal contraceptive implant are longer than approved by the FDA, and patient-clinician shared decision-making should be used. Subcutaneous depot medroxyprogesterone acetate, 104 mg, a newer formulation with prefilled syringes, can be safely self-administered every 13 weeks. Because bone density loss appears to be reversible, the American College of Obstetricians and Gynecologists recommends considering use of depot medroxyprogesterone acetate beyond two years despite an FDA boxed warning about increased fracture risk. Testosterone does not prevent pregnancy but is safe to use with hormonal contraception; thus, transgender and gender-diverse patients with a uterus can be offered the full range of contraceptive options.
In the United States, 45% of pregnancies in 2011 were unintended.1 Primary care clinicians are uniquely situated to provide holistic contraceptive care in the context of a patient's medical comorbidities, social circumstance, and gender identity. This article addresses recent updates on the topic of contraception and answers common questions for clinicians. Table 1 includes comprehensive family planning resources provided by the Centers for Disease Control and Prevention (CDC).2
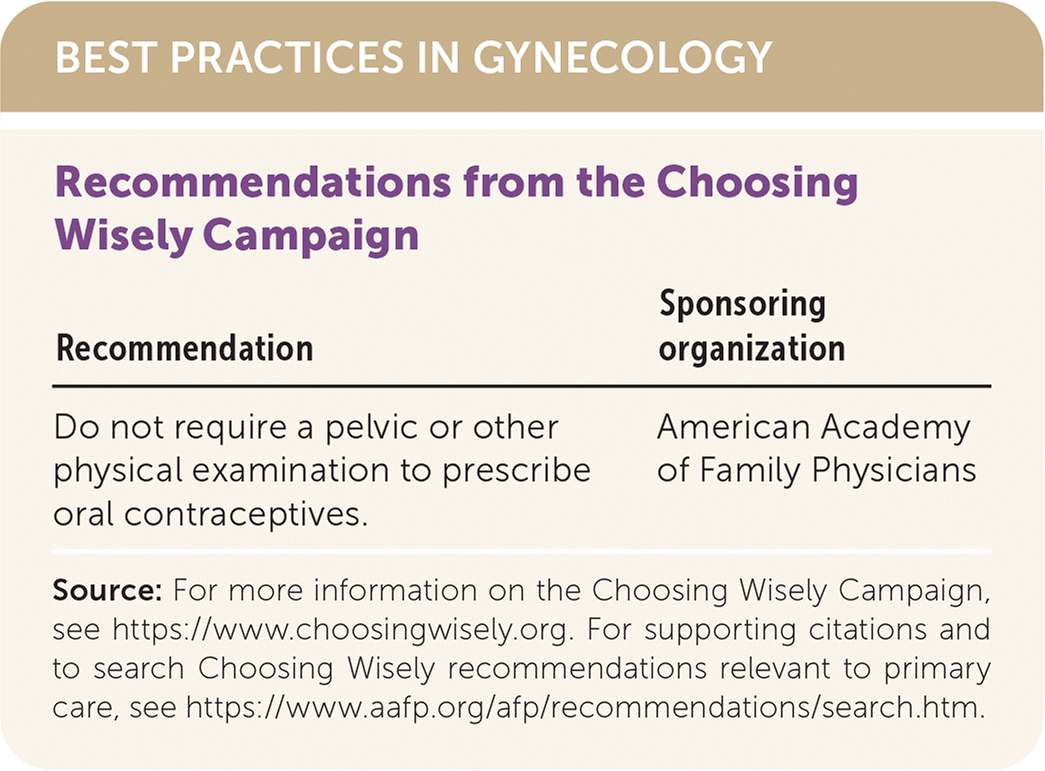
| Recommendation | Sponsoring organization |
|---|---|
| Do not require a pelvic or other physical examination to prescribe oral contraceptives. | American Academy of Family Physicians |
| Resource | Examples of content |
|---|---|
| Guidelines for providing family planning services: https://www.cdc.gov/reproductivehealth/contraception/qfp.htm | Helping patients achieve pregnancy Contraceptive services Preconception counseling Pregnancy testing and counseling Sexually transmitted infection screening Preventive health services Screening for breast or cervical cancer Conducting quality improvement of family planning metrics |
| Guidelines for initiating and managing specific contraceptionmethods: https://www.cdc.gov/reproductivehealth/contraception/mmwr/spr/summary.html | How to be reasonably certain an individual is not pregnant (Table 5) Contraceptive options Examinations and testing Follow-up planning Managing common adverse effects of contraception Initiating a contraception method Switching to a different method Postpartum and postabortion use |
| U.S. Medical Eligibility Criteria for Contraceptive Use, 2016: https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html | Contraception options are evaluated based on common patient characteristics and medical conditions and are rated for safety according to an evidence review |
| Sexually transmitted infection treatment guidelines, 2021 (updated for drug resistance patterns): https://www.cdc.gov/std/treatment-guidelines/STI-Guidelines-2021.pdf | Treatment options for patients who have or are at risk of sexually transmitted infections |
| Patient resource on contraception: https://www.cdc.gov/reproductivehealth/contraception/unintendedpregnancy | Risks and benefits of common contraceptives |
| Smartphone application summarizing contraception recommendations: https://www.cdc.gov/reproductivehealth/contraception/contraception-app.html | Summary of U.S. Medical Eligibility Criteria for Contraceptive Use and selected U.S. practice recommendations |
What Forms of Emergency Contraception Are Effective?
The copper intrauterine device (IUD; Paragard) is the most effective intervention for emergency contraception when placed within 120 hours of unprotected intercourse. A large single study suggests that the levonorgestrel-releasing intrauterine system, 52 mg, (Mirena, Liletta) is similarly effective. Oral ulipristal (Ella); oral levonorgestrel, 1.5 mg, (Plan B One-Step); and the Yuzpe method are also effective if started within 120 hours. Emergency contraception should be started as soon as possible.3–5 Oral emergency contraception may have decreased effectiveness with increasing body mass index. Table 2 summarizes emergency contraception options.5–8
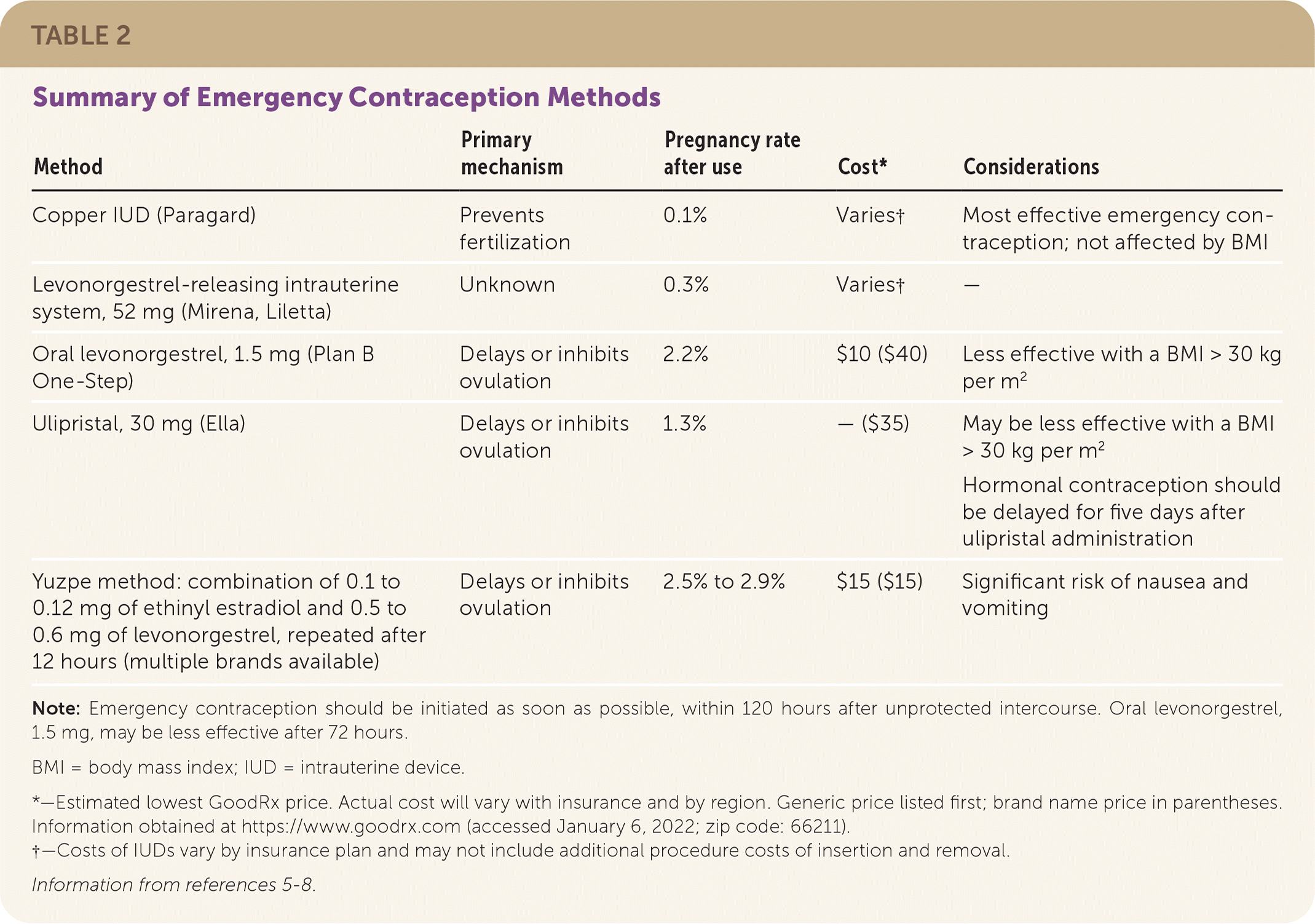
| Method | Primary mechanism | Pregnancy rate after use | Cost* | Considerations |
|---|---|---|---|---|
| Copper IUD (Paragard) | Prevents fertilization | 0.1% | Varies† | Most effective emergency contraception; not affected by BMI |
| Levonorgestrel-releasing intrauterine system, 52 mg (Mirena, Liletta) | Unknown | 0.3% | Varies† | — |
| Oral levonorgestrel, 1.5 mg (Plan B One-Step) | Delays or inhibits ovulation | 2.2% | $10 ($40) | Less effective with a BMI > 30 kg per m2 |
| Ulipristal, 30 mg (Ella) | Delays or inhibits ovulation | 1.3% | — ($35) | May be less effective with a BMI > 30 kg per m2 Hormonal contraception should be delayed for five days after ulipristal administration |
| Yuzpe method: combination of 0.1 to 0.12 mg of ethinyl estradiol and 0.5 to 0.6 mg of levonorgestrel, repeated after 12 hours (multiple brands available) | Delays or inhibits ovulation | 2.5% to 2.9% | $15 ($15) | Significant risk of nausea and vomiting |
EVIDENCE SUMMARY
When placed within 120 hours after unprotected intercourse, the copper IUD is 99.9% effective at preventing pregnancy.9 A recent study suggests that the levonorgestrel-releasing intrauterine system, 52 mg, is similarly effective.6 In 317 patients who received the levonorgestrel-releasing intrauterine system, 52 mg, within 120 hours of unprotected intercourse, only one pregnancy occurred. This was similar to the group using the copper IUD.6 Both methods offer reliable long-term contraception after placement, high continuation rates, high user satisfaction, and consistent effectiveness regardless of body mass index.4–6
Ulipristal is the most effective oral emergency contraceptive, with a 1.3% pregnancy rate when started within 120 hours. Oral levonorgestrel, 1.5 mg, is slightly less effective than ulipristal with a 2.5% pregnancy rate.7 Oral levonorgestrel, 1.5 mg, is approximately twice as effective if given within 72 hours than when given at 72 to 120 hours, and it is less effective for obese patients.7,8 Pregnancy rates may also increase for obese patients using ulipristal, but data are conflicting.8 The Yuzpe method uses a combination of oral contraceptives (0.1 to 0.12 mg of ethinyl estradiol and 0.5 to 0.6 mg of levonorgestrel) repeated after 12 hours. It is a low-cost, widely available method but is less effective than oral levonorgestrel, 1.5 mg, or ulipristal.7 The Yuzpe method is associated with high rates of nausea and vomiting.4,5,7
Oral emergency contraception does not affect an existing pregnancy, and a pregnancy test is unnecessary before use.3,5 Because ulipristal may interact with the progestin component of hormonal contraceptives, it is recommended to wait at least five days before starting hormonal contraception after using ulipristal to preserve emergency contraception effectiveness.4 Hormonal contraception may be started on the same day as oral levonorgestrel, 1.5 mg, administration.5 If a patient does not have a withdrawal bleed within three weeks of using oral emergency contraception, a pregnancy test should be performed.4
Are Fertility Awareness Methods of Contraception Effective?
Fertility awareness methods, which predict timing of ovulation so that intercourse can be avoided, have varying rates of effectiveness that are comparable to barrier and withdrawal methods.10 Smartphone-based applications that aid in fertility awareness are advertised as improving effectiveness but may not be subject to peer review.
EVIDENCE SUMMARY
Fertility awareness methods track fertility intervals and physiologic changes, including body temperature, presence and consistency of cervical mucus, and urinary hormone excretion, to predict ovulation for the purpose of avoiding intercourse during that time.5,11,12 These methods were previously reviewed in American Family Physician (https://www.aafp.org/afp/2012/1115/p924). Although 4% of women report using fertility awareness methods, clinicians may not counsel patients on these methods because of perceived ineffectiveness, lack of training, insufficient time, or poor reimbursement.12,13
A systematic review that excluded low-quality studies found that typical use of fertility awareness methods results in 2.0 to 33.6 pregnancies per 100 person-years (Table 311), depending on the method used, and is comparable to typical condom use and the withdrawal method.5,11 Smart-phone applications that aid in fertility awareness improve adherence and ease of use, but effectiveness studies may not be subject to peer review.14,15 The U.S. Food and Drug Administration (FDA) permitted marketing of the smart-phone applications Natural Cycles and Clue Birth Control to prevent pregnancy, noting a 6.5% failure rate over one year with typical use.16,17
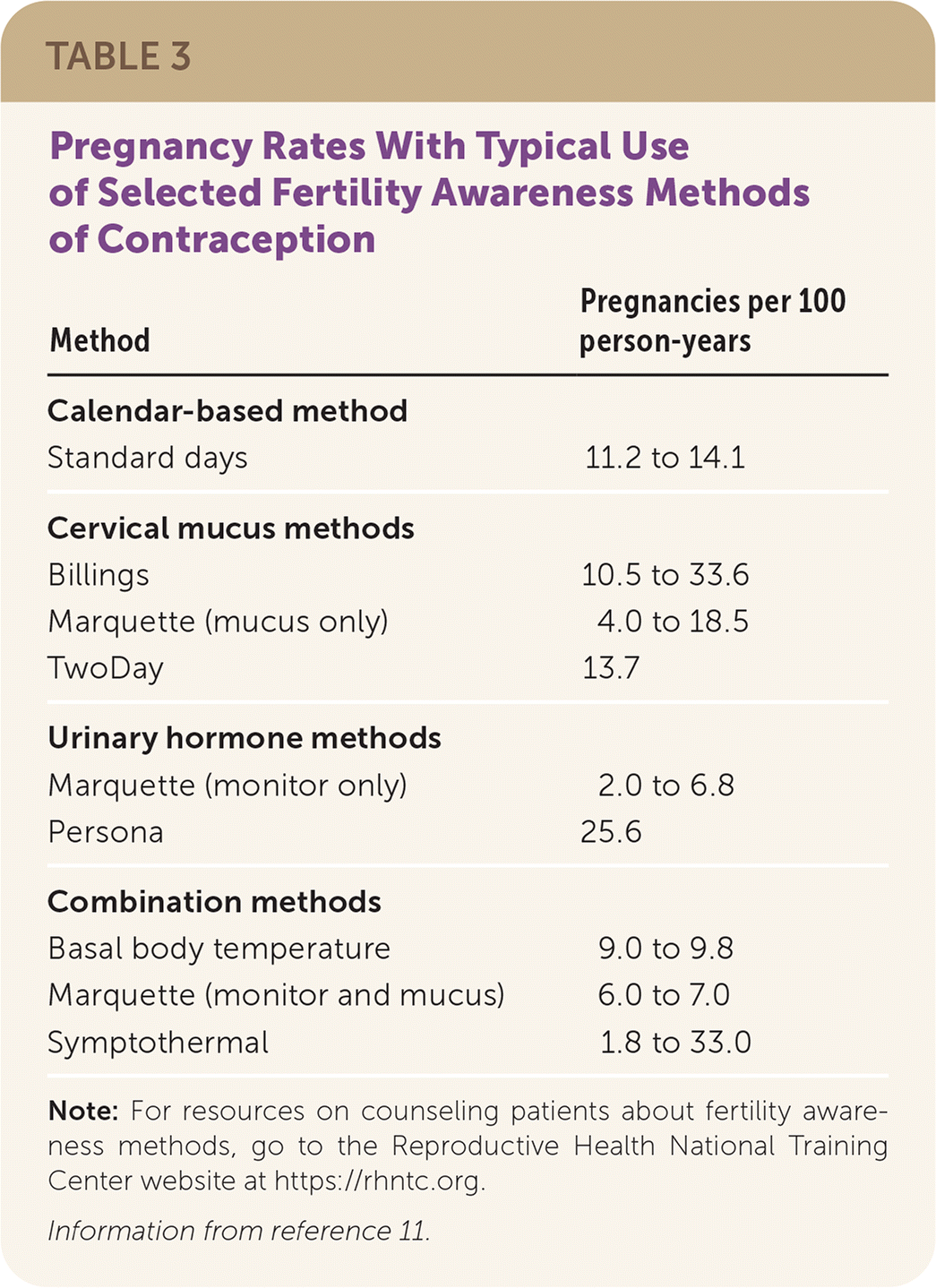
| Method | Pregnancies per 100 person-years |
|---|---|
| Calendar-based method | |
| Standard days | 11.2 to 14.1 |
| Cervical mucus methods | |
| Billings | 10.5 to 33.6 |
| Marquette (mucus only) | 4.0 to 18.5 |
| TwoDay | 13.7 |
| Urinary hormone methods | |
| Marquette (monitor only) | 2.0 to 6.8 |
| Persona | 25.6 |
| Combination methods | |
| Basal body temperature | 9.0 to 9.8 |
| Marquette (monitor and mucus) | 6.0 to 7.0 |
| Symptothermal | 1.8 to 33.0 |
Fertility awareness methods should be avoided if patients have irregular menstrual cycles or partners who do not support abstinence or alternative contraceptive methods during fertile windows. They are a safe and reasonable alternative for patients who prefer to avoid hormonal contraception.11
What Contraceptive Methods Are Less Safe for People With Migraines?
Migraine with aura is associated with an increased risk of ischemic stroke, whereas the risk for those who have migraine without aura is less clear.18,19 Use of combined hormonal contraceptives, including oral contraceptives, transdermal patch, or vaginal ring, in patients who have migraine with aura appears to increase stroke risk.19–21 The CDC's U.S. Medical Eligibility Criteria for Contraceptive Use (USMEC) recommend against prescribing combined hormonal contraceptives for patients who have migraine with aura.20
EVIDENCE SUMMARY
In patients with migraines, use of combined hormonal contraceptives has been associated with a two- to fourfold increase in ischemic stroke risk; however, most studies do not differentiate between migraine subtypes.19,20 Early studies suggested that the increased risk of ischemic stroke was common to all migraines, but more recent meta-analyses suggest the increased risk is limited to those who have migraine with aura.18,22
In a case-control study using a nationwide database, the presence of migraine with aura and the use of combined hormonal contraceptives appear to increase risk of ischemic stroke, and risks may be additive.21 Risk of stroke in patients who had migraine without aura was approximately twice as high as patients without migraine, regardless of combined hormonal contraceptive use.21 Another study suggests that migraine with aura is associated with 1.5 times greater odds of ischemic stroke compared with no migraine, regardless of combined hormonal contraceptive use.23 Although ischemic stroke risk from the use of combined hormonal contraceptives is theorized to be dependent on estrogen dose, evidence is limited.19 The CDC and American College of Obstetricians and Gynecologists recommend avoiding the use of estrogen-containing contraceptives in patients who have migraine with aura but note that clear evidence of harm is limited.20,24
All patients with headaches should be evaluated for migraines and the presence of aura.24 Migraine subtypes were discussed previously in American Family Physician (https://www.aafp.org/afp/2019/0101/p17.html and https://www.aafp.org/afp/2020/0401/p419.html).
The USMEC has four categories of medical eligibility for contraceptive use.20 Combined hormonal contraceptives are USMEC category 2 (i.e., advantages generally outweigh the risks) for patients who have migraines without aura, including menstrual migraine, and category 4 (i.e., unacceptable health risks) for those who have migraines with aura. Nonhormonal and progestin-only contraceptives are category 1 (i.e., no restrictions for use). Category 3 indicates that the theoretical or proven risks usually outweigh advantages. More information on the USMEC recommendations can be found at https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html.
How Long Does Long-Acting Reversible Contraception Remain Effective?
Data support continued contraceptive benefit from the levonorgestrel-releasing intrauterine system, 52 mg, for eight years; the copper IUD for 12 years; and the etonogestrel subdermal contraceptive implant (Nexplanon) for five years.25,26 In 2021, the FDA approved use of one levonorgestrel-releasing intrauterine system, 52 mg, (Mirena) for eight years to prevent pregnancy. Extending use of other forms of long-acting reversible contraception (LARC) beyond the FDA-approved duration requires shared decision-making for off-label use.
EVIDENCE SUMMARY
LARC, including the levonorgestrel-releasing intrauterine system, copper IUD, and etonogestrel subdermal implant, reliably prevents pregnancy with typical use (less than one pregnancy per 100 patient-years over 12 months).5 LARC is user independent and cost-effective, resulting in high patient satisfaction and high rates of continuation for 12 months. LARC can be used safely and effectively in adolescents and nulliparous patients.5,27–29 Table 4 summarizes the key characteristics of LARC methods.5,25,26,30,31
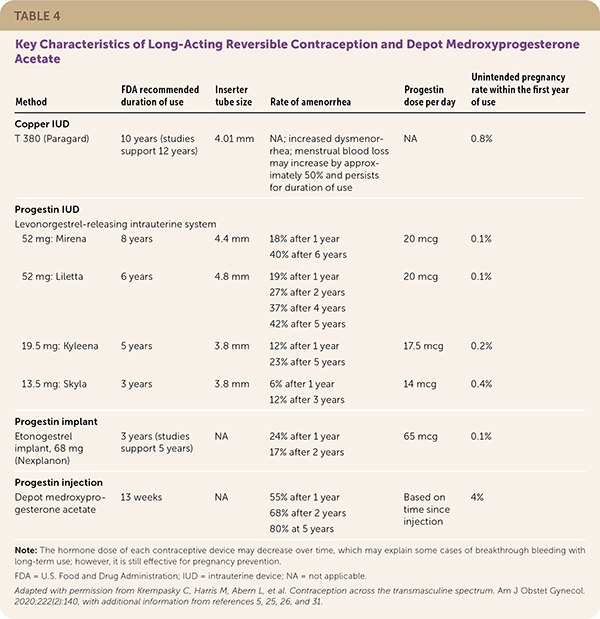
| Method | FDA recommended duration of use | Inserter tube size | Rate of amenorrhea | Progestin dose per day | Unintended pregnancy rate within the first year of use |
|---|---|---|---|---|---|
| Copper IUD | |||||
| T 380 (Paragard) | 10 years (studies support 12 years) | 4.01 mm | NA; increased dysmenorrhea; menstrual blood loss may increase by approximately 50% and persists for duration of use | NA | 0.8% |
| Progestin IUD | |||||
| Levonorgestrel-releasing intrauterine system | |||||
| 52 mg: Mirena | 7 years | 4.4 mm | 18% after 1 year 40% after 6 years | 20 mcg | 0.1% |
| 52 mg: Liletta | 6 years | 4.8 mm | 19% after 1 year 27% after 2 years 37% after 4 years 42% after 5 years | 20 mcg | 0.1% |
| 19.5 mg: Kyleena | 5 years | 3.8 mm | 12% after 1 year 23% after 5 years | 17.5 mcg | 0.2% |
| 13.5 mg: Skyla | 3 years | 3.8 mm | 6% after 1 year 12% after 3 years | 14 mcg | 0.4% |
| Progestin implant | |||||
| Etonogestrel implant, 68 mg (Nexplanon) | 3 years (studies support 5 years) | NA | 24% after 1 year 17% after 2 years | 65 mcg | 0.1% |
| Progestin injection | |||||
| Depot medroxyprogesterone acetate | 13 weeks | NA | 55% after 1 year 68% after 2 years 80% at 5 years | Based on time since injection | 4% |
The levonorgestrel-releasing intrauterine system, 52 mg, was initially certified by the FDA for five years of use, whereas the copper IUD was initially certified for 10 years. However, studies demonstrate that the levonorgestrel-releasing intrauterine system continues to be highly effective for at least eight years, and the copper IUD is effective for at least 12 years.25,32–34
A systematic review showed that the levonorgestrel-releasing intrauterine system had a lower pregnancy rate during the sixth and seventh year of use than in the first year.25 Small studies support longer duration of effectiveness for the levonorgestrel-releasing intrauterine system, with no pregnancies in 776 patients over 10 years in one study.32 The FDA recently certified Mirena for eight years and Liletta for six years of use to prevent pregnancy.33,34
The copper IUD also appears to be effective during extended use. In two studies with 314 patients, use of the copper IUD for 12 years instead of 10 years did not result in additional pregnancies.25 The FDA has not addressed extending the duration of copper IUD use.
The etonogestrel subdermal contraceptive implant is approved by the FDA for three years of use, but data suggest that it remains effective for at least five years. Serum etonogestrel levels have been shown to remain above contraceptive levels at five years.35 In observational studies with more than 1,000 participants, no pregnancies occurred between three and five years of use.26,35
The CDC recommends that clinicians be reasonably certain that a patient is not pregnant before IUD placement (Table 5).4 This does not necessarily require a negative pregnancy test, which may be inaccurate in early pregnancy. The growth in telemedicine may increase the opportunity for counseling patients on the use of LARC options. Counseling should be based on noncoercive shared decision-making through patient-centered, reproductive-justice principles.36,37

| No signs/symptoms of pregnancy and any one of the following criteria: |
| 7 days or less since the start of normal menses |
| No sexual intercourse since start of last normal menses |
| Correct and consistent use of reliable contraception |
| 7 days or less since spontaneous or induced abortion |
| Within 4 weeks postpartum |
| Breastfeeding nearly exclusively (at least 85% of feedings), presence of amenorrhea, and less than 6 months since delivery |
Can Depot Medroxyprogesterone Acetate Be Self-Administered Subcutaneously? What Are the Effects on Bone Mineral Density?
Subcutaneous depot medroxyprogesterone acetate (DeposubQ Provera) can be safely and effectively self-administered, potentially leading to fewer missed injections and unintended pregnancies. Depot medroxyprogesterone acetate may lead to loss of bone mineral density, which should be a consideration in those with relevant medical conditions.19,20,38,39
EVIDENCE SUMMARY
Self-Administration of Subcutaneous Injections. Similar to 150-mg intramuscular depot medroxyprogesterone acetate (Depo-Provera), 104-mg subcutaneous depot medroxyprogesterone acetate is a progestin-only contraceptive that can be administered every 12 to 15 weeks.39–43 However, the subcutaneous method uses a smaller needle and injection volume.39–43 Self-administration of subcutaneous depot medroxyprogesterone acetate has been shown to be effective in clinical trials. In a meta-analysis of 3,851 participants, self-administered subcutaneous injections improved adherence compared with injections administered by a clinician, with comparable pregnancy rates and adverse effects other than injection site reactions.31
Subcutaneous depot medroxyprogesterone is certified by the FDA for administration by a clinician. Self-administration is off-label use.43 To reduce COVID-19-related barriers to care, some states have expanded telemedicine coverage for self-administration of subcutaneous depot medroxyprogesterone acetate.44 The World Health Organization and CDC recommend self-administration of the subcutaneous injections as an additional approach to deliver injectable contraception to improve health equity, particularly among youth and marginalized populations.40,43
Subcutaneous administration of depot medroxyprogesterone acetate has the same indications, risks, and benefits as intramuscular administration.4,20,43 Instructions for self-administration of the subcutaneous injection are available at https://www.reproductiveaccess.org/wp-content/uploads/2017/08/2021-04-english-depo-sub-q.pdf.
Effect of Depot Medroxyprogesterone Acetate on Bone Mineral Density. Unlike other commonly used contraceptives, depot medroxyprogesterone acetate decreases pituitary gonadotropin secretion, leading to reduced estrogen production and possibly loss of bone mineral density.38 In 2004, the FDA issued a boxed warning indicating that fracture risk may increase after two years of use.38 Subsequent studies have shown that the loss of bone mineral density is substantially recoverable after stopping the medication, suggesting that there may be less risk of fracture with prolonged use than previously thought.20,38,45,46
The American College of Obstetricians and Gynecologists recommends shared decision-making for extending the use of depot medroxyprogesterone acetate beyond two years in healthy patients, including review of the FDA warning and subsequent evidence; however, concerns about bone mineral density should not prevent continuing use beyond two years.38 According to the CDC, there are no restrictions on the use of depot medroxyprogesterone acetate in healthy patients between 18 and 45 years of age.20 Depot medroxyprogesterone acetate may have a higher risk of bone mineral density effects for patients younger than 18 years and older than 45 years, and when conditions such as cystic fibrosis, inflammatory bowel disease, or multiple sclerosis predispose the patient to lower bone mineral density.20 For these special conditions, the CDC suggests that advantages of depot medroxyprogesterone acetate generally outweigh the risks.20
What Are Contraception Considerations in Transgender and Gender-Diverse People With a Uterus?
Transgender and gender-diverse people with a uterus, including those assigned female sex at birth who have transgender or nonbinary masculine-spectrum identity or expression, can safely be offered the full range of contraceptive options, including emergency contraception. Contraception is effective for pregnancy prevention or menstrual suppression with gender-affirming hormone treatments.30,47 Unintended pregnancies occur in transgender people taking testosterone, demonstrating that testosterone is not effective for contraception.
EVIDENCE SUMMARY
Even in the presence of amenorrhea, testosterone treatment does not prevent ovulation.48 In addition to the family planning benefit, contraception has a safety function for transmasculine patients. Testosterone is a known teratogen that requires a washout period to prevent abnormal genital development in the fetus if the patient becomes pregnant.30 Because pregnancy or menstruation may cause significant distress for transmasculine people, regular reproductive health counseling, including family planning and preferences for fertility preservation, is important.36 Counseling should include discussing sexual behavior that may result in pregnancy.47,49
Combining testosterone therapy with contraceptives containing estrogen does not appear to increase the risk of venous thromboembolism.30 Transmasculine people may prefer to avoid oral contraceptives because of their associations with cisgender women and their perceived, but unlikely, feminizing effects.30,47,50 Continuous use of contraceptives containing estrogen effectively suppresses uterine bleeding, and these contraceptives are easily discontinued when desired. Daily oral norethindrone acetate can suppress menses but has not been proven effective for contraception.30
LARC is approximately 20 times more effective than daily oral contraceptives, making them appropriate options for transmasculine people.27 IUD placement may be uncomfortable and distressful for transgender patients, which can be attenuated by anticipatory counseling, anxiolytics, sedation, smaller speculums, and short-term vaginal estradiol (Estrace) before the procedure.30 Among progestin-only options, depot medroxyprogesterone acetate and the levonorgestrel-releasing intrauterine system, 52 mg, are most likely to lead to long-term amenorrhea.30 Oophorectomy and hysterectomy are considered medically necessary for trans-masculine people who desire permanent sterilization.47,51,52
When a transmasculine person with amenorrhea uses emergency contraception, a pregnancy test should be performed within four weeks. If a patient experiences bleeding after having amenorrhea or develops new pelvic pain, earlier evaluation including testing for pregnancy and sexually transmitted infections should be performed.30
To mitigate stigma and barriers to care, clinicians can create welcoming environments by using gender-neutral language (e.g., people who menstruate, external pelvic area, chest instead of breasts, and absorbent products instead of menstrual pads or tampons).30,53 Creating an inclusive clinical practice was discussed previously in American Family Physician (https://www.aafp.org/afp/2018/1201/p645.html).
Data Sources: A PubMed search was completed using the MeSH terms natural family planning methods, long-acting reversible contraception, hormonal contraception, migraine disorders, contraception, postcoital, or transgender persons. The reference lists of three cited manuscripts, guidelines by the American College of Obstetricians and Gynecologists and the World Health Organization, and two relevant reviews were searched for additional studies of interest. Other queries included Essential Evidence Plus, UpToDate, and the Cochrane Database of Systematic Reviews. Search dates: April 6 to June 6, 2021, and February 18, 2022.
Editor's Note: In August 2022, the U.S. Food and Drug Administration extended the recommended duration of use for the levonorgestrel-releasing intrauterine system, 52 mg, (Mirena) to eight years based on a demonstration of more than 99% effectiveness between six and eight years of use. This change is not reflected in the print edition of this article.—Sumi Sexton, MD, Editor-in-Chief
The views expressed in this publication are those of the authors and do not reflect the official policy or position of the Departments of the Navy and Air Force, the Department of Defense, or the U.S. government.