
Am Fam Physician. 2023;107(4):370-381
Published online March 10, 2023.
The content in this article is current as of March 6, 2023.
Author disclosure: No relevant financial relationships.
New COVID-19 variants of concern continue to develop. Incubation period, transmissibility, immune escape, and treatment effectiveness differ by variants of concern. Physicians should be aware that the characteristics of the predominant variants of concern determine aspects of diagnosis and treatment. Multiple testing modalities exist; the most appropriate testing strategy varies depending on the clinical scenario, with factors of test sensitivity, turnaround time, and the expertise required for specimen collection. Three types of vaccines are available in the United States, and all people six months and older should be encouraged to receive one because vaccination is effective in reducing the incidence of and hospitalizations and deaths associated with COVID-19. Vaccination may also reduce the incidence of post-acute sequelae of SARS-CoV-2 infection (i.e., long COVID). Consider medications, such as nirmatrelvir/ritonavir, as first-line treatment for eligible patients diagnosed with COVID-19 unless logistical or supply constraints occur. National Institutes of Health guidelines and local health care partner resources can be used to determine eligibility. Long-term health effects of having COVID-19 are under investigation.
This article summarizes the prevention, diagnosis, and treatment of COVID-19, with a particular emphasis on outpatient management.
| Three vaccine types are available in the United States, and all people six months and older should be encouraged to receive one because vaccination effectively reduces the incidence of and hospitalizations and deaths associated with COVID-19. |
| The effective reproduction number (infections per index case) of the Omicron variant of concern was 2.7 times higher than the Delta variant of concern. As a result, transmission increased eight- to 10-fold compared with the ancestral strain among children from birth to three years during the Omicron variant of concern. |
| A large U.S. Department of Veterans Affairs study found substantial long-term (12 months and beyond) cardiovascular risks associated with COVID-19 (e.g., dysrhythmias, heart failure, inflammatory heart disease, thromboembolic disease). |
Epidemiology
COVID-19 is caused by an enveloped single-stranded RNA novel coronavirus, SARS-CoV-2. To date, several variants of concern (i.e., Alpha, Beta, Delta, Gamma, and Omicron) have emerged because of viral mutations, and additional variants will likely continue to emerge.1
The incidence of COVID-19 in the United States varies geographically. For up-to-date information about cases, deaths, hospitalizations, and vaccinations, see national data trackers (e.g., https://covid.cdc.gov/covid-data-tracker/#data-tracker-home [updated daily]).
The prevalence of COVID‐19 for patients testing positive if they have symptoms of illness varies from 5% to 38% (median = 17%).2
Spread from asymptomatic infected persons and airborne transmission (not just on respiratory droplets) contributes to the high incidence and prevalence of SARS-CoV-2 infections.3
Racial and ethnic minorities experience higher rates of COVID-19 and consequently more hospitalizations and deaths compared with non-Hispanic White populations in the United States. These disparities are likely due to increased exposure, economic inequities, and lack of health care access.4
The effective reproduction number (infections per index case) of the Omicron variant was 2.7 times higher than the Delta variant.5 As a result, transmission increased eight- to 10-fold compared with the ancestral strain among children from birth to three years of age during the Omicron variant's phase.6 Reinfection rates also increased.7,8
Mean incubation periods were shorter for the Omicron vs. Alpha variant (approximately three days compared with almost five days, respectively).9
Prevention
Vaccination was effective in reducing incidence of COVID-19 early in the pandemic and continues to be effective at preventing severe illness, hospitalization, and death.10
Three types of vaccines against SARS-CoV-2 are available in the United States: two mRNA vaccines, an adenovirus vector, and a new protein subunit vaccine11–16 (eTable A). The mRNA vaccines are preferred over the adenovirus vector vaccine because of rare serious and potentially life-threatening clotting events, particularly among women who are 18 to 49 years of age, associated with the adenovirus vector.16
During the Omicron variant wave, receiving two or three doses of an mRNA COVID-19 vaccine was associated with a 90% reduction in risk for COVID-19–associated invasive mechanical ventilation or death17 (Table 111–23); however, protection against symptomatic infection wanes rapidly. The clinical effectiveness of the bivalent mRNA vaccine is not yet known; clinical trials are underway. Clinical trials are also underway for the new NVX-CoV2373 (Novavax) vaccine for the Omicron variant.20
COVID-19 vaccination is recommended during pregnancy because of the risks of pregnancy complications associated with COVID-19 and the absence of data suggesting potential harm.24 One recent systematic review demonstrated reduced infection and hospitalization in pregnant patients who had been vaccinated, with no adverse perinatal, fetal, or neonatal outcomes.25
Quarantining has been effective in reducing COVID-19 incidence and mortality, and combining quarantine with other public health measures (e.g., travel restrictions, mandated social distancing) increased effectiveness.26 However, with widespread immunization and improved treatments, quarantine is no longer recommended for people who have been exposed to COVID-19.27
Mask mandates have proven effective in reducing COVID-19 incidence.28 One study found a 23% lower incidence of COVID-19 in school districts with mask mandates compared with those without.29 The American Academy of Family Physicians supports the use of high-quality face masks to prevent transmission of COVID-19.30
Stay-at-home orders early in the pandemic reduced morbidity and decreased COVID-19 incidence and deaths.31
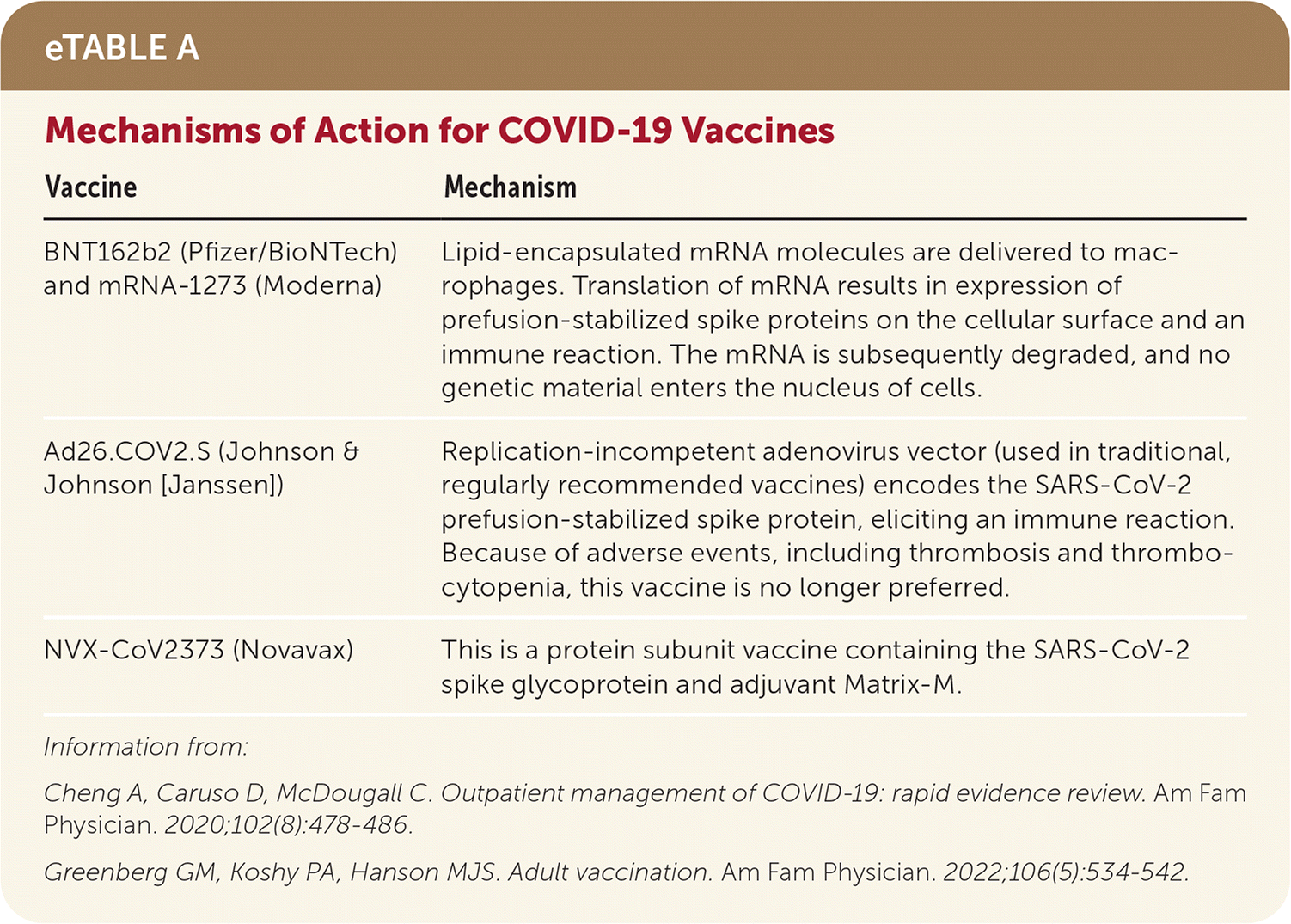
| Vaccine | Mechanism |
|---|---|
| BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna) | Lipid-encapsulated mRNA molecules are delivered to macrophages. Translation of mRNA results in expression of prefusion-stabilized spike proteins on the cellular surface and an immune reaction. The mRNA is subsequently degraded, and no genetic material enters the nucleus of cells. |
| Ad26.COV2.S (Johnson & Johnson [Janssen]) | Replication-incompetent adenovirus vector (used in traditional, regularly recommended vaccines) encodes the SARS-CoV-2 prefusion-stabilized spike protein, eliciting an immune reaction. Because of adverse events, including thrombosis and thrombocytopenia, this vaccine is no longer preferred. |
| NVX-CoV2373 (Novavax) | This is a protein subunit vaccine containing the SARS-CoV-2 spike glycoprotein and adjuvant Matrix-M. |
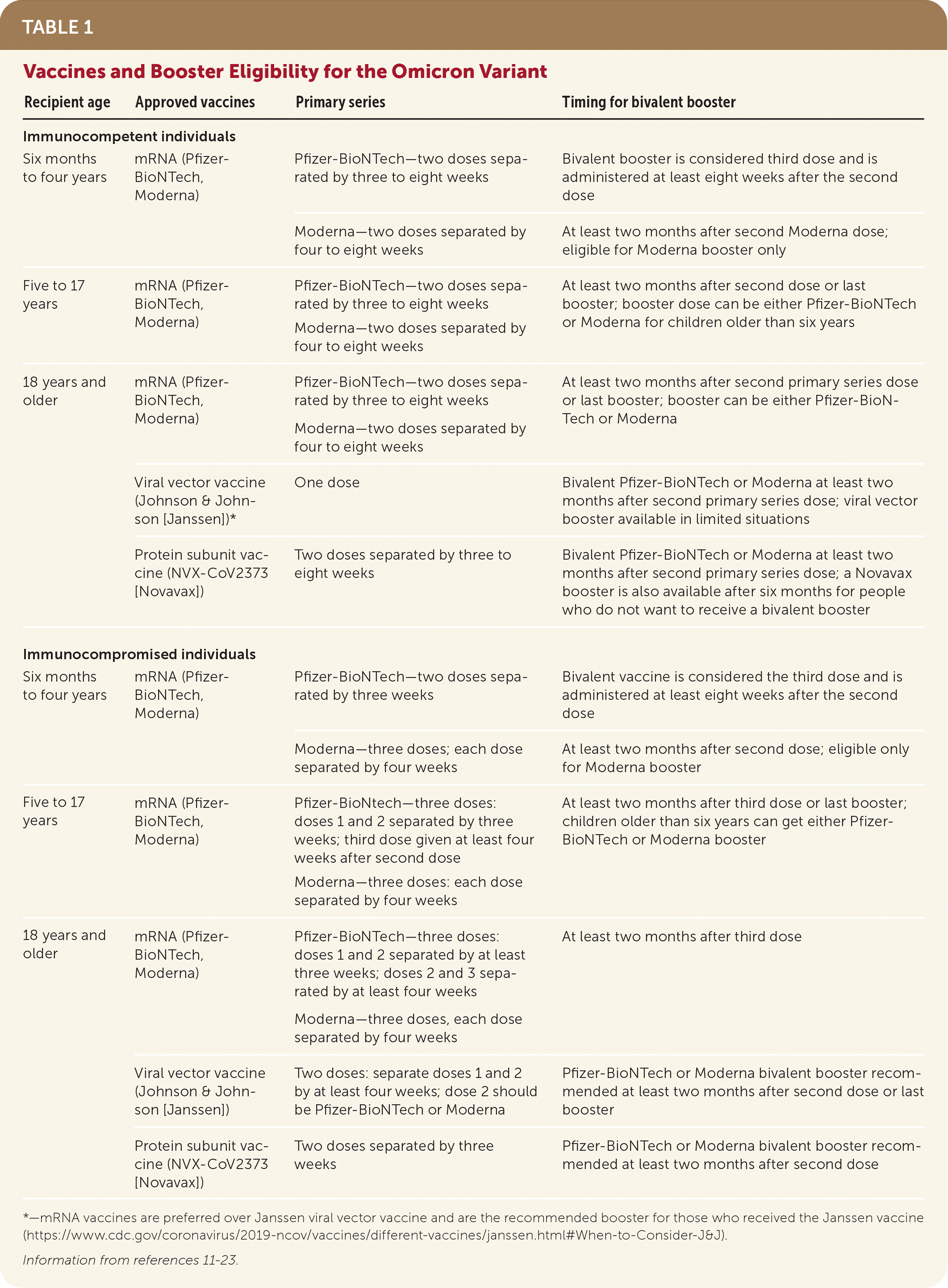
| Recipient age | Approved vaccines | Primary series | Timing for bivalent booster |
|---|---|---|---|
| Immunocompetent individuals | |||
| Six months to four years | mRNA (Pfizer-BioNTech, Moderna) | Pfizer-BioNTech—two doses separated by three to eight weeks | Bivalent booster is considered third dose and is administered at least eight weeks after the second dose |
| Moderna—two doses separated by four to eight weeks | At least two months after second Moderna dose; eligible for Moderna booster only | ||
| Five to 17 years | mRNA (Pfizer-BioNTech, Moderna) | Pfizer-BioNTech—two doses separated by three to eight weeks Moderna—two doses separated by four to eight weeks | At least two months after second dose or last booster; booster dose can be either Pfizer-BioNTech or Moderna for children older than six years |
| 18 years and older | mRNA (Pfizer-BioNTech, Moderna) | Pfizer-BioNTech—two doses separated by three to eight weeks Moderna—two doses separated by four to eight weeks | At least two months after second primary series dose or last booster; booster can be either Pfizer-BioNTech or Moderna |
| Viral vector vaccine (Johnson & Johnson [Janssen])* | One dose | Bivalent Pfizer-BioNTech or Moderna at least two months after second primary series dose; viral vector booster available in limited situations | |
| Protein subunit vaccine (NVX-CoV2373 [Novavax]) | Two doses separated by three to eight weeks | Bivalent Pfizer-BioNTech or Moderna at least two months after second primary series dose; a Novavax booster is also available after six months for people who do not want to receive a bivalent booster | |
| Immunocompromised individuals | |||
| Six months to four years | mRNA (Pfizer-BioNTech, Moderna) | Pfizer-BioNTech—two doses separated by three weeks | Bivalent vaccine is considered the third dose and is administered at least eight weeks after the second dose |
| Moderna—three doses; each dose separated by four weeks | At least two months after second dose; eligible only for Moderna booster | ||
| Five to 17 years | mRNA (Pfizer-BioNTech, Moderna) | Pfizer-BioNtech—three doses: doses 1 and 2 separated by three weeks; third dose given at least four weeks after second dose Moderna—three doses: each dose separated by four weeks | At least two months after third dose or last booster; children older than six years can get either Pfizer-BioNTech or Moderna booster |
| 18 years and older | mRNA (Pfizer-BioNTech, Moderna) | Pfizer-BioNTech—three doses: doses 1 and 2 separated by at least three weeks; doses 2 and 3 separated by at least four weeks Moderna—three doses, each dose separated by four weeks | At least two months after third dose |
| Viral vector vaccine (Johnson & Johnson [Janssen]) | Two doses: separate doses 1 and 2 by at least four weeks; dose 2 should be Pfizer-BioNTech or Moderna | Pfizer-BioNTech or Moderna bivalent booster recommended at least two months after second dose or last booster | |
| Protein subunit vaccine (NVX-CoV2373 [Novavax]) | Two doses separated by three weeks | Pfizer-BioNTech or Moderna bivalent booster recommended at least two months after second dose | |
Diagnosis
The diagnosis of COVID-19 is made clinically and is supported by laboratory results and radiographic findings. COVID-19 should be suspected in patients with symptoms, close contact with known cases, or in the setting of active SARS-CoV-2 transmission in the community.32
SARS-CoV-2 infection is possible after recent vaccination, and postvaccination symptoms should not be attributed to vaccine adverse effects without considering new diagnosis of SARS-CoV-2 infection.33
The differential diagnosis for COVID-19 pneumonia includes the following32:
○ Other viral upper and lower respiratory tract infection
○ Influenza
○ Community-acquired pneumonia (bacterial or viral)
○ Streptococcal pharyngitis
○ Acute pulmonary edema
○ Chronic obstructive pulmonary disease exacerbation
○ Hantavirus pulmonary syndrome
○ Interstitial lung disease
○ Opportunistic pulmonary infection
○ Pulmonary embolism
SIGNS AND SYMPTOMS
The most common presenting symptoms of patients who have COVID-19 include pharyngitis, cough, fatigue, and headache.34,35
More than 90 different symptoms of COVID-19 have been reported, and their prevalence has changed with different variants of concern.2 Symptoms include fever, myalgias, headache, rhinorrhea, chest pain/tightness, shortness of breath or difficulty breathing, and diarrhea.34–36 Patients may have a wide range of symptoms representing a spectrum of mild to severe illness.
Predictors of severe disease include increasing age, comorbidities, myocardial injury, low platelet count, and high levels of C-reactive protein.32,37
The diagnostic value of common symptoms of COVID-19 is highly variable. In variants in early 2020, cough (62.4%), fever (37.6%), and sore throat (31.0%) were the most sensitive, whereas the symptoms best at ruling in COVID-19 when present were anosmia or ageusia (positive likelihood ratio [LR+] = 5.0), anosmia alone (LR+ = 4.6), or ageusia alone (LR+ = 3.1). Anosmia and ageusia have become less frequent and sore throat more frequent since emergence of the Omicron variant.38
Multisystem inflammatory syndrome is a rare but serious complication of SARS-CoV-2 in children. Signs include prolonged fever, rash, conjunctivitis, and clinically severe illness with multiorgan involvement, including gastrointestinal and cardiac systems. Central nervous system involvement has been reported. The diagnosis is supported by laboratory confirmation of inflammation, evidence of recent SARS-CoV-2 infection or exposure, and no other obvious microbial cause of inflammation.39,40
DIAGNOSTIC TESTING
All individuals who have suspected SARS-CoV-2 infection or known exposure should be tested. Testing can also be considered before an indoor event or gathering, especially one involving immunocompromised or unvaccinated people.41
Multiple testing modalities are available for COVID-1942–44 (Table 245). When deciding which testing modality to use, physicians should consider setting, type of sample, turnaround time, and pretest probability.
Antibody testing should be reserved for epidemiologic purposes.46
Testing asymptomatic people after an exposure is recommended based on the median viral incubation period, about three days with the Omicron variant.
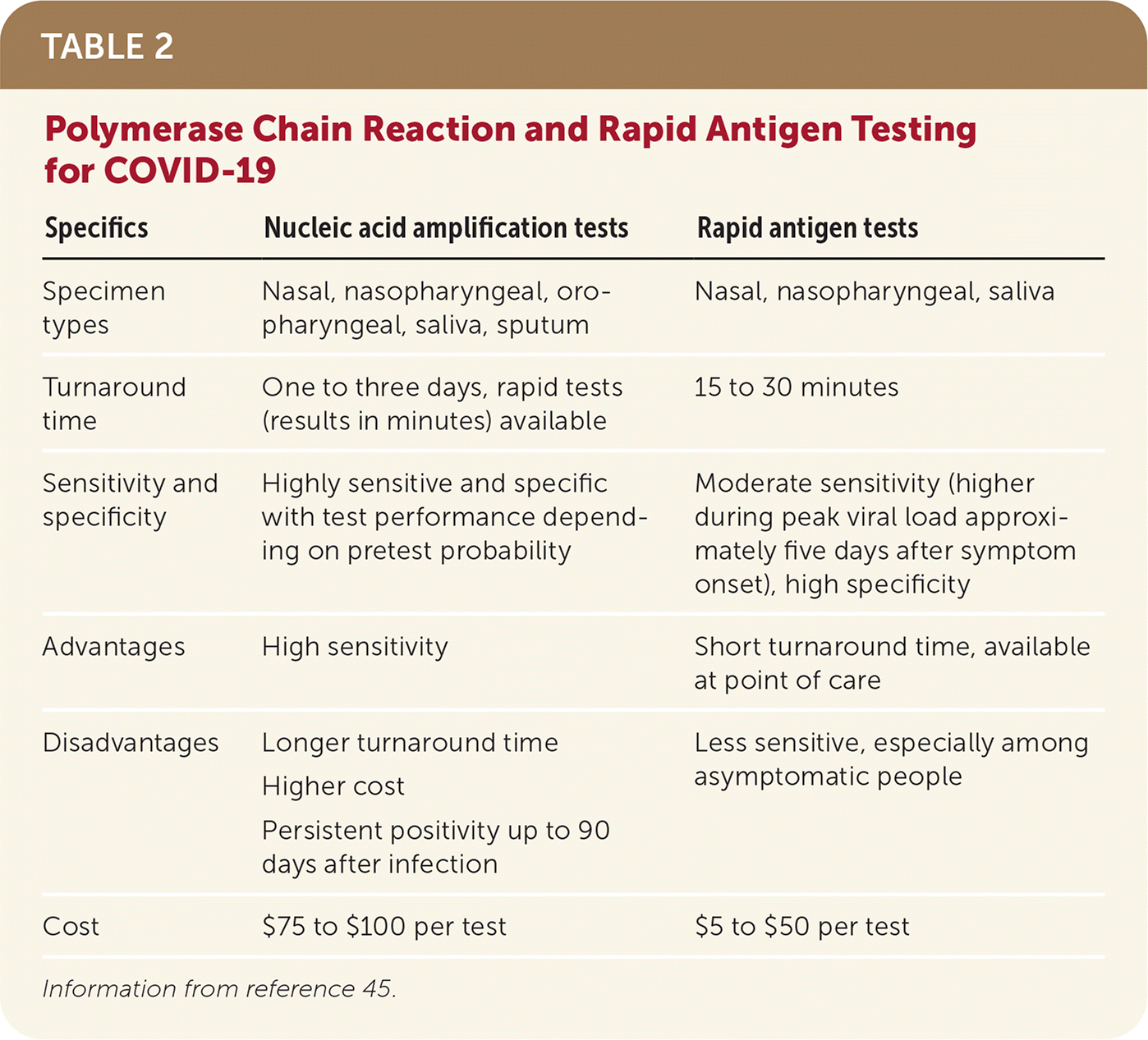
| Specifics | Nucleic acid amplification tests | Rapid antigen tests |
|---|---|---|
| Specimen types | Nasal, nasopharyngeal, oropharyngeal, saliva, sputum | Nasal, nasopharyngeal, saliva |
| Turnaround time | One to three days, rapid tests (results in minutes) available | 15 to 30 minutes |
| Sensitivity and specificity | Highly sensitive and specific with test performance depending on pretest probability | Moderate sensitivity (higher during peak viral load approximately five days after symptom onset), high specificity |
| Advantages | High sensitivity | Short turnaround time, available at point of care |
| Disadvantages | Longer turnaround time Higher cost Persistent positivity up to 90 days after infection | Less sensitive, especially among asymptomatic people |
| Cost | $75 to $100 per test | $5 to $50 per test |
Treatment
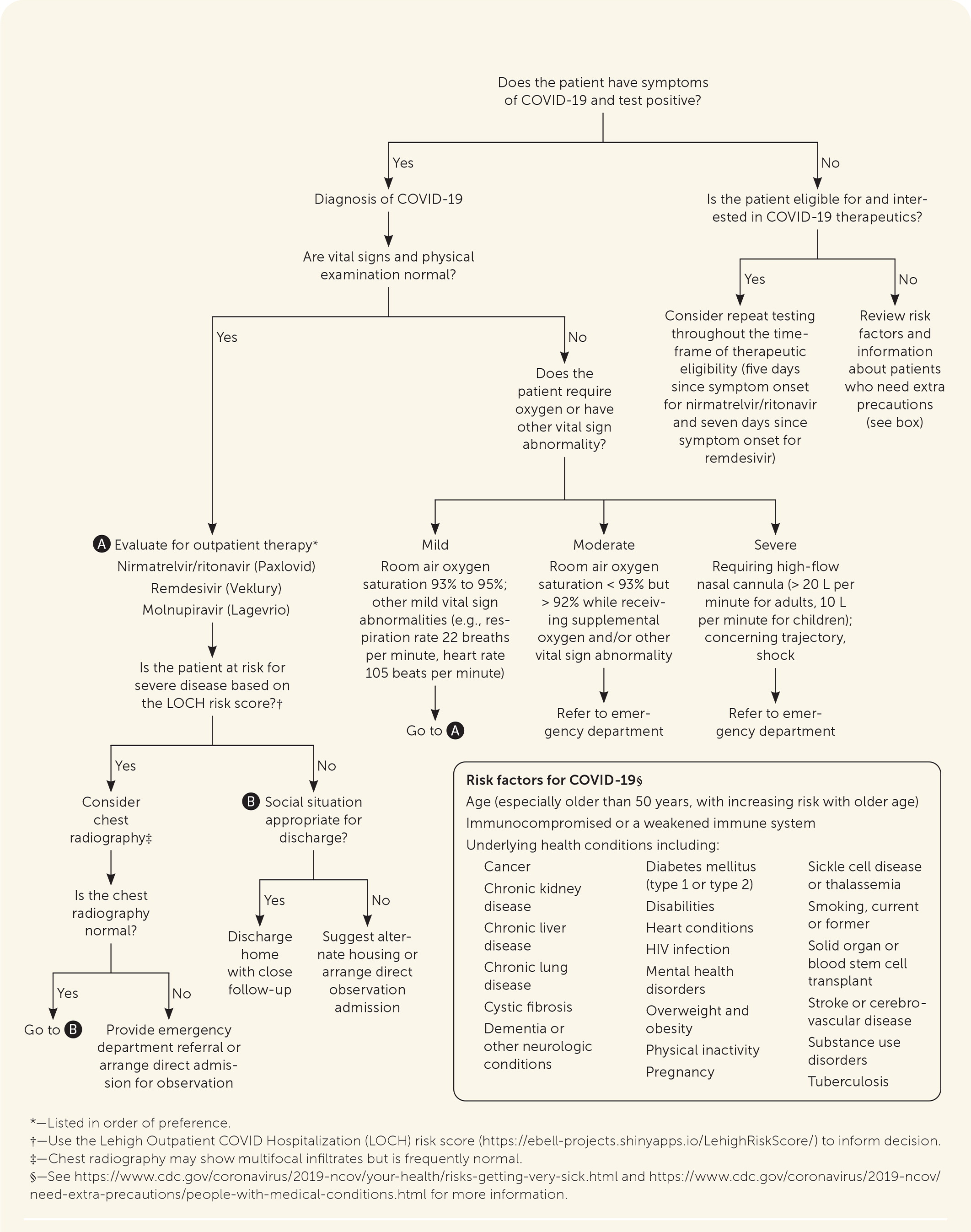
All patients should be assessed for treatment eligibility (Figure 147–52). Therapies are prioritized for those at highest risk of progression to severe disease.53 Symptom management is indicated for patients who do not meet criteria for drug therapy.54
Individuals diagnosed with COVID-19 should receive education about isolating, separating from other people at home, and avoiding travel and public transportation. Individuals should also tell close contacts that they may have been exposed to COVID-19.55
Current treatment approaches, including eligibility criteria, are curated by the National Institutes of Health.54 Recommended treatments may vary depending on their effectiveness against the predominant variant of concern. Current first-line therapies are oral nirmatrelvir/ritonavir (Paxlovid) and injectable remdesivir (Veklury), and second-line treatment is molnupiravir (Lagevrio); Table 3 provides more information.56–64
The American Academy of Family Physicians advises against all non–U.S. Food and Drug Administration-approved treatments, such as hydroxychloroquine, colchicine (Colcrys), lopinavir/ritonavir, and ivermectin (Stromectol).65–68 Systemic corticosteroids are not recommended for outpatients or for hospitalized patients who do not require supplemental oxygen.54
The World Health Organization recommends that infants born to patients with COVID-19 not be separated from their mothers and that these mothers initiate and continue breastfeeding because the risk of separation is greater than the risk of transmission.69
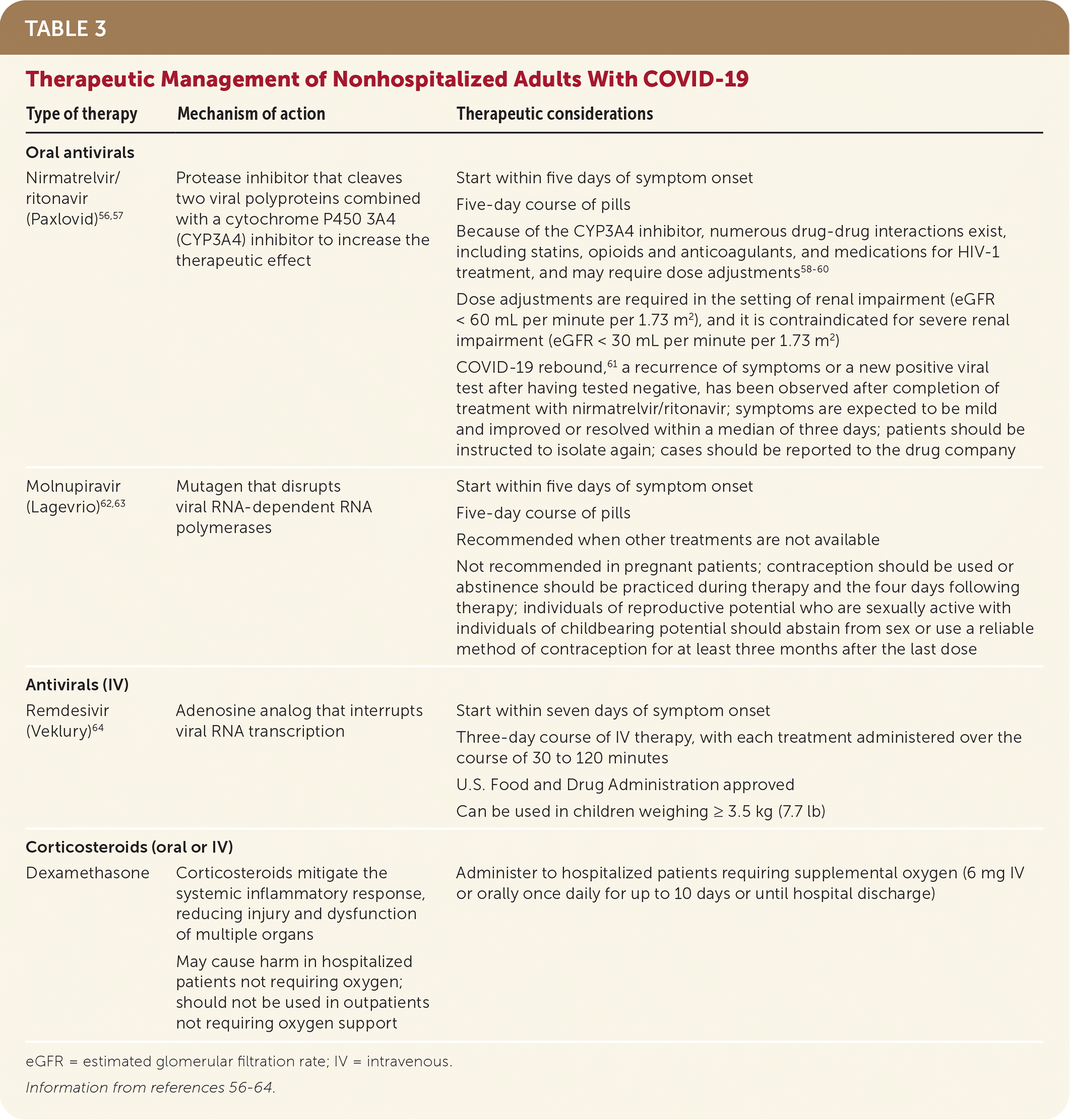
| Type of therapy | Mechanism of action | Therapeutic considerations |
|---|---|---|
| Oral antivirals | ||
| Nirmatrelvir/ritonavir (Paxlovid)56,57 | Protease inhibitor that cleaves two viral polyproteins combined with a cytochrome P450 3A4 (CYP3A4) inhibitor to increase the therapeutic effect | Start within five days of symptom onset Five-day course of pills Because of the CYP3A4 inhibitor, numerous drug-drug interactions exist, including statins, opioids and anticoagulants, and medications for HIV-1 treatment, and may require dose adjustments58–60 Dose adjustments are required in the setting of renal impairment (eGFR < 60 mL per minute per 1.73 m2), and it is contraindicated for severe renal impairment (eGFR < 30 mL per minute per 1.73 m2) COVID-19 rebound,61 a recurrence of symptoms or a new positive viral test after having tested negative, has been observed after completion of treatment with nirmatrelvir/ritonavir; symptoms are expected to be mild and improved or resolved within a median of three days; patients should be instructed to isolate again; cases should be reported to the drug company |
| Molnupiravir (Lagevrio)62,63 | Mutagen that disrupts viral RNA-dependent RNA polymerases | Start within five days of symptom onset Five-day course of pills Recommended when other treatments are not available Not recommended in pregnant patients; contraception should be used or abstinence should be practiced during therapy and the four days following therapy; individuals of reproductive potential who are sexually active with individuals of childbearing potential should abstain from sex or use a reliable method of contraception for at least three months after the last dose |
| Antivirals (IV) | ||
| Remdesivir (Veklury)64 | Adenosine analog that interrupts viral RNA transcription | Start within seven days of symptom onset Three-day course of IV therapy, with each treatment administered over the course of 30 to 120 minutes U.S. Food and Drug Administration approved Can be used in children weighing ≥ 3.5 kg (7.7 lb) |
| Corticosteroids (oral or IV) | ||
| Dexamethasone | Corticosteroids mitigate the systemic inflammatory response, reducing injury and dysfunction of multiple organs May cause harm in hospitalized patients not requiring oxygen; should not be used in outpatients not requiring oxygen support | Administer to hospitalized patients requiring supplemental oxygen (6 mg IV or orally once daily for up to 10 days or until hospital discharge) |
DETERMINING OUTPATIENT DISPOSITION
The appropriate disposition of patients with symptomatic COVID-19 can be guided by vital signs, physical examination findings, respiratory rate, oxygen saturation, social considerations, and risk factors,47–50,70 as outlined in Figure 1.47–52
The Lehigh Outpatient COVID Hospitalization (LOCH) risk score (https://ebell-projects.shinyapps.io/LehighRiskScore/) can be used to predict the risk of hospitalization based on age, comorbidities, and the presence of dyspnea. It has been validated during the Omicron variant phase.71
Consider referral to the emergency department for further evaluation in patients who are high risk and selectively among moderate-risk patients. There should be a low threshold for in-person evaluation of older people and those with high-risk medical conditions.
SUPPORTIVE CARE
Approximately 80% of patients with COVID-19 have mild illness not requiring medical intervention or hospitalization.32
Consider home monitoring respiratory rate and/or oxygen saturation in patients who are assessed to be at risk for severe disease or who are at moderate risk using the LOCH score.
Moderate-risk patients should be instructed to seek medical care immediately if they develop any of the following:
○ Trouble breathing or shortness of breath
○ Persistent pain or pressure in the chest
○ New confusion
○ Inability to wake or stay awake
○ Pale, gray- or blue-colored skin, lips, or nail beds, depending on skin tone
○ Any symptoms that are severe or concerning
Self-care includes staying hydrated, resting, and taking over-the-counter medications, such as acetaminophen.47,54
ANTIVIRAL THERAPY
Antiviral treatments for use in the outpatient setting include oral treatments, nirmatrelvir/ritonavir or molnupiravir, as well as injectable remdesivir.
Nirmatrelvir/ritonavir taken orally is the recommended first-line treatment and is shown to reduce the risk of progression to severe COVID-19 by 89% in unvaccinated patients if prescribed within five days of symptom onset.72 A recent study confirmed the benefit of nirmatrelvir/ritonavir in older vaccinated patients.73 Dysgeusia and diarrhea are known adverse effects.
○ Modifications to drug therapy (e.g., statins) are required because of metabolism by cytochrome P450 3A4. Dose adjustment is required in patients with moderate renal impairment (estimated glomerular filtration rate of 30 mL or more to less than 60 mL per minute per 1.73 m2). Nirmatrelvir/ritonavir is not recommended for use in patients with severe renal impairment (estimated glomerular filtration rate less than 30 mL per minute per 1.73 m2) or with severe hepatic impairment (Child-Pugh Class C).58
○ Nirmatrelvir/ritonavir should be considered for pregnant patients with mild to moderate symptoms, particularly if one or more additional risk factors are present.56 There are no available human data on the use of nirmatrelvir during pregnancy. The mechanism of action and the results of animal studies suggest that nirmatrelvir/ritonavir can be used safely in pregnancy.56 Ritonavir has been used extensively during pregnancy in people who have HIV.74 Breastfeeding is not a contraindication for use of nirmatrelvir/ritonavir.
Remdesivir administered intravenously over three days is considered an alternative to nirmatrelvir/ritonavir. Studies showed an 87% lower risk of hospitalization or death than placebo among nonhospitalized patients at high risk of severe disease.64 Remdesivir is the only antiviral treatment approved for children 12 years or younger or weighing at least 3.5 kg (7.7 lb) to 40 kg (88 lb), although there is insufficient evidence to recommend routine use in children younger than 12 years.75
In adults with mild or moderate symptomatic COVID-19 who are older than 60 years or who have a risk factor for severe disease, the risk of hospitalization or death was significantly reduced with molnupiravir, with a number needed to treat of 33.62,63 It is well tolerated, but the risk of mutagenesis limits its acceptability. Pregnant patients are ineligible. People with childbearing potential should be advised to use contraception or practice abstinence during therapy and the four days following therapy. Individuals with reproductive potential who are sexually active with individuals of childbearing potential should abstain from sex or use a reliable method of contraception for at least three months after the last dose. Similar to the primary study of nirmatrelvir/ritonavir, the randomized trial included only unvaccinated patients.62
MONOCLONAL ANTIBODIES
Monoclonal antibody treatments directed against the spike protein have been effective in the past but are not effective with current variants of concern.47,70
Monoclonal treatments benefit from fewer drug-drug interactions and effectiveness when administered within seven days of symptom onset; however, they must be intravenously administered.
There are insufficient data to evaluate the risks of monoclonal antibodies in people who are pregnant or breastfeeding.76
Preexposure prophylaxis with tixagevimab/cilgavimab (Evusheld) has been given to those with compromised immune systems not expected to develop antibodies after vaccination, but the drug is not effective against current variants of concern.77
Prognosis
Hospitalization rates of patients with the Omicron variant ranged from 3.2 per 100,000 among people 12 to 34 years of age who have been vaccinated to 233.3 per 100,000 of those 66 years and older who are unvaccinated (Table 4).78
The infection fatality ratio from COVID-19 has been estimated to range from 0.002% for those nine years or younger to 9.3% for those 80 years and older.79
The mRNA vaccines are highly effective in preventing hospitalizations and reducing disease severity and mortality.78,80,81
Predictors of mortality and/or severe disease include age older than 50 years, several comorbidities, and examination and laboratory findings71,82 (Table 582).
Pregnant patients with COVID-19 are at risk for more serious illness, as well as adverse pregnancy and neonatal outcomes.83 The risk of severe COVID-19 infection in pregnancy is higher in patients who have diabetes mellitus, obesity, preeclampsia, or a history of smoking.84 There is some evidence of vertical transmission of COVID-19, although the risk is still poorly understood.83,85
Acute complications of COVID-19 with high likelihood include cardiovascular complications, thrombosis, acute kidney injury, and post-intensive care syndrome for those treated in the intensive care unit.86
The long-term health effects of COVID-19 are under investigation. One large U.S. Department of Veterans Affairs study found substantial long-term (12 months and beyond) cardiovascular risks associated with COVID-19 (e.g., dysrhythmias, inflammatory heart disease, heart failure, thromboembolic disease).87
Post-acute sequelae of SARS-CoV-2 infection (i.e., long COVID) is a common complication of COVID-19; the duration of these symptoms varies, and the ultimate prognosis is as yet unknown for many patients.88–90 Those hospitalized had much higher rates (73% to 93%) than those who were not hospitalized (23%).89,91 Studies suggest that vaccination before SARS-CoV-2 infection reduces the risk of subsequent long COVID.92,93 Risk of long COVID increased in patients with older age, allergies, or an increasing number of comorbidities.94
Fatigue was the most common persistent issue, with shortness of breath for those who were hospitalized and anosmia for those who were not hospitalized.95
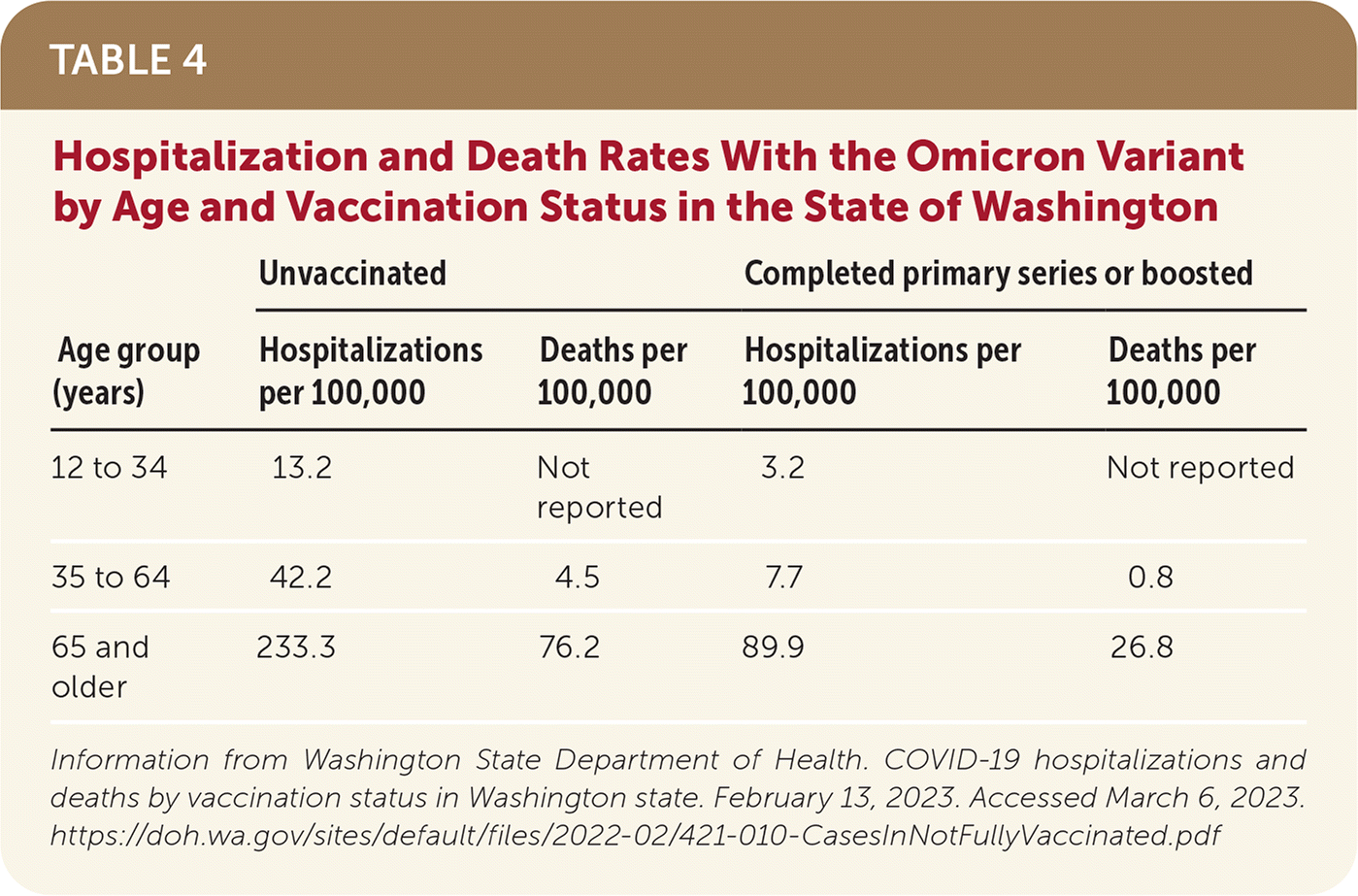
| Age group (years) | Unvaccinated | Completed primary series or boosted | ||
|---|---|---|---|---|
| Hospitalizations per 100,000 | Deaths per 100,000 | Hospitalizations per 100,000 | Deaths per 100,000 | |
| 12 to 34 | 13.2 | Not reported | 3.2 | Not reported |
| 35 to 64 | 42.2 | 4.5 | 7.7 | 0.8 |
| 65 and older | 233.3 | 76.2 | 89.9 | 26.8 |
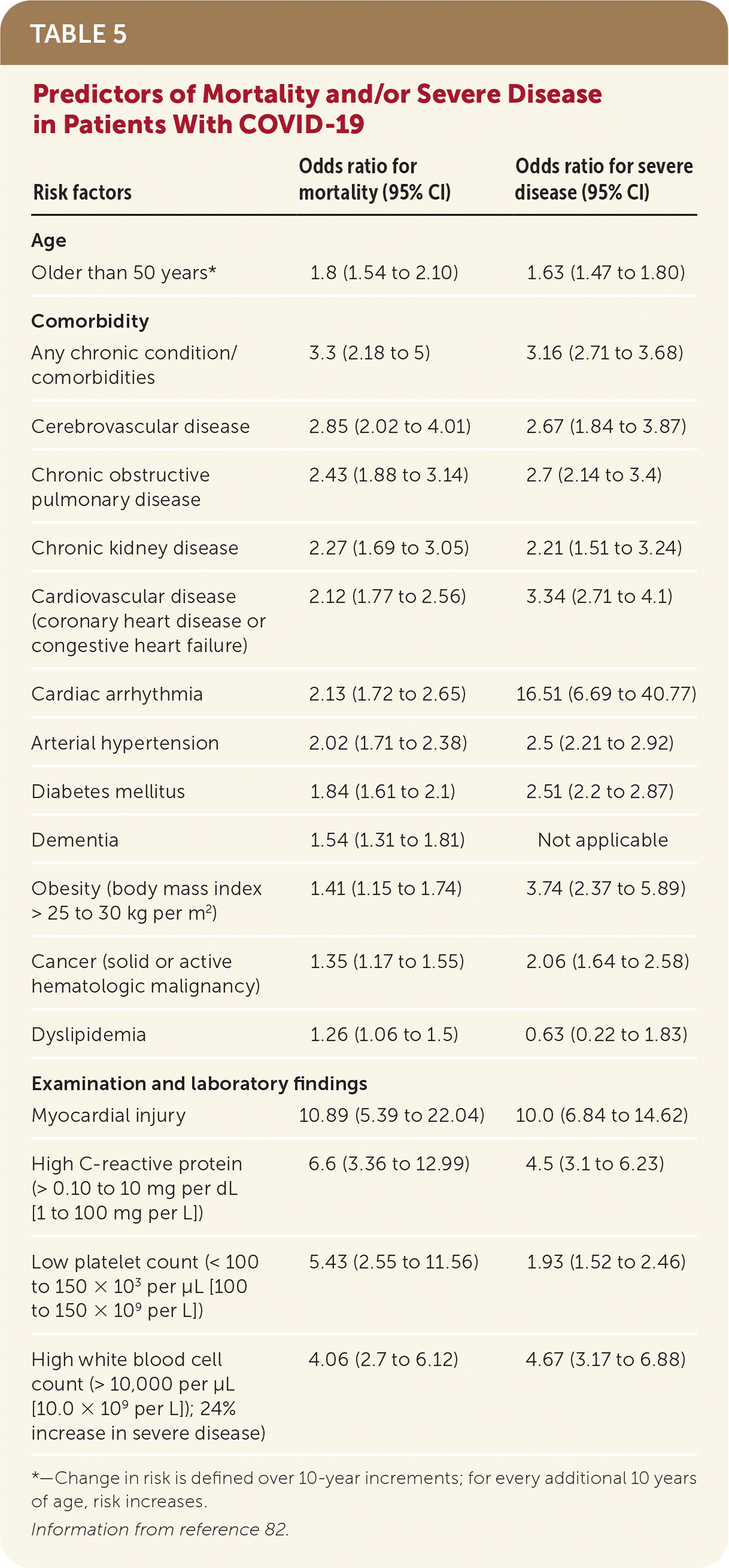
| Risk factors | Odds ratio for mortality (95% CI) | Odds ratio for severe disease (95% CI) |
|---|---|---|
| Age | ||
| Older than 50 years* | 1.8 (1.54 to 2.10) | 1.63 (1.47 to 1.80) |
| Comorbidity | ||
| Any chronic condition/comorbidities | 3.3 (2.18 to 5) | 3.16 (2.71 to 3.68) |
| Cerebrovascular disease | 2.85 (2.02 to 4.01) | 2.67 (1.84 to 3.87) |
| Chronic obstructive pulmonary disease | 2.43 (1.88 to 3.14) | 2.7 (2.14 to 3.4) |
| Chronic kidney disease | 2.27 (1.69 to 3.05) | 2.21 (1.51 to 3.24) |
| Cardiovascular disease (coronary heart disease or congestive heart failure) | 2.12 (1.77 to 2.56) | 3.34 (2.71 to 4.1) |
| Cardiac arrhythmia | 2.13 (1.72 to 2.65) | 16.51 (6.69 to 40.77) |
| Arterial hypertension | 2.02 (1.71 to 2.38) | 2.5 (2.21 to 2.92) |
| Diabetes mellitus | 1.84 (1.61 to 2.1) | 2.51 (2.2 to 2.87) |
| Dementia | 1.54 (1.31 to 1.81) | Not applicable |
| Obesity (body mass index > 25 to 30 kg per m2) | 1.41 (1.15 to 1.74) | 3.74 (2.37 to 5.89) |
| Cancer (solid or active hematologic malignancy) | 1.35 (1.17 to 1.55) | 2.06 (1.64 to 2.58) |
| Dyslipidemia | 1.26 (1.06 to 1.5) | 0.63 (0.22 to 1.83) |
| Examination and laboratory findings | ||
| Myocardial injury | 10.89 (5.39 to 22.04) | 10.0 (6.84 to 14.62) |
| High C-reactive protein (> 0.10 to 10 mg per dL [1 to 100 mg per L]) | 6.6 (3.36 to 12.99) | 4.5 (3.1 to 6.23) |
| Low platelet count (< 100 to 150 × 103 per μL [100 to 150 × 109 per L]) | 5.43 (2.55 to 11.56) | 1.93 (1.52 to 2.46) |
| High white blood cell count (> 10,000 per μL [10.0 × 109 per L]); 24% increase in severe disease) | 4.06 (2.7 to 6.12) | 4.67 (3.17 to 6.88) |
This article updates a previous article on this topic by Cheng, et al.96
Data Sources: Search terms included COVID, COVID-19, and SARS-CoV-2. A literature review was performed using PubMed, Essential Evidence Plus, and the COVID-19 daily research briefs on the American Family Physician website. Search dates: March 2022 through February 2023.
