Am Fam Physician. 2023;107(6):623-630
Related Letter to the Editor: Addressing Mood Disorders in Infertility Care
Related Letter to the Editor: Longer Menstrual Cycle and Infertility Evaluation
Related editorial: Addressing Disparities in Infertility Care
Patient information: See related handout on infertility.
Author disclosure: No relevant financial relationships.
Infertility is the inability to achieve a pregnancy after 12 months of regular, unprotected sexual intercourse. Evaluation and treatment are recommended earlier than 12 months when risk factors for infertility exist, if the female partner is 35 years or older, and in the setting of nonheterosexual partnerships. A comprehensive medical history and physical examination emphasizing the thyroid, breast, and pelvic areas should be performed to help direct diagnosis and treatment. Causes of infertility in females include uterine and tubal factors, ovarian reserve, ovulatory dysfunction, obesity, and hormone-related disorders. Common male factor infertility issues include abnormal semen, hormonal disorders, and genetic abnormalities. Semen analysis is recommended for the initial assessment of the male partner. Evaluation of the female should include assessment of the uterus and fallopian tubes with ultrasonography or hysterosalpingography when indicated. Laparoscopy, hysteroscopy, or magnetic resonance imaging may be needed to evaluate for endometriosis, leiomyomas, or evidence of a previous pelvic infection. Treatment with ovulation induction agents, intrauterine insemination, in vitro fertilization, donor sperm or eggs, or surgery may be necessary. Unexplained male and female infertility can be treated with intrauterine insemination or in vitro fertilization. Limiting alcohol intake, avoiding tobacco and illicit drug use, consuming a profertility diet, and losing weight (if obese) may improve pregnancy success rates.
Infertility is the inability to achieve a pregnancy after 12 months of regular, unprotected sexual intercourse; more broadly, infertility describes the impairment of a person's capacity to reproduce as an individual or with their partner.1 Infertility affects between 8% and 12% of couples of reproductive age worldwide, with some variation based on geographic location.2 In the United States, 12.2% of females 15 to 49 years of age have received infertility services.3
Primary infertility is having never achieved a pregnancy. Secondary infertility is the inability to achieve a pregnancy after a previous pregnancy. Both contribute significantly to infertility worldwide.2
This article focuses on infertility in opposite-sex partners; however, same-sex couples and others in nonheterosexual partnerships also require evaluation and management of fertility issues. For this review, female and male refer to the sex assigned at birth.
Epidemiology
Globally, 48 million couples and 186 million individuals are affected by infertility.4 The National Survey of Family Growth shows that 19.4% of currently married women in the United States who are 15 to 49 years of age have had zero births and are considered infertile, and 26% of women 15 to 49 years of age have impaired fecundity.3–5 The percentage of women with infertility is lower among those 15 to 29 years of age and increases with age. Infertility was formally designated a disease in 2009 by the World Health Organization and the American Medical Association6; however, access and cost can be substantial barriers to receiving infertility care.7
Etiology
Timing of Evaluation
The American Society for Reproductive Medicine and the American College of Obstetricians and Gynecologists recommend that the female partner's age determine the time frame for evaluation.10,11 If the female is younger than 35 years, evaluation and treatment should begin after 12 months of regular, unprotected intercourse. Evaluation should occur after six months if the female partner is between 35 and 40 years of age. If the female partner is older than 40 years or has any condition considered high risk for infertility (e.g., known tubal disease, pelvic inflammatory disease, previous ectopic pregnancy), immediate evaluation and treatment are recommended1,9,10,12 (Table 19,10). In the setting of nonheterosexual partnerships, immediate evaluation is also recommended.
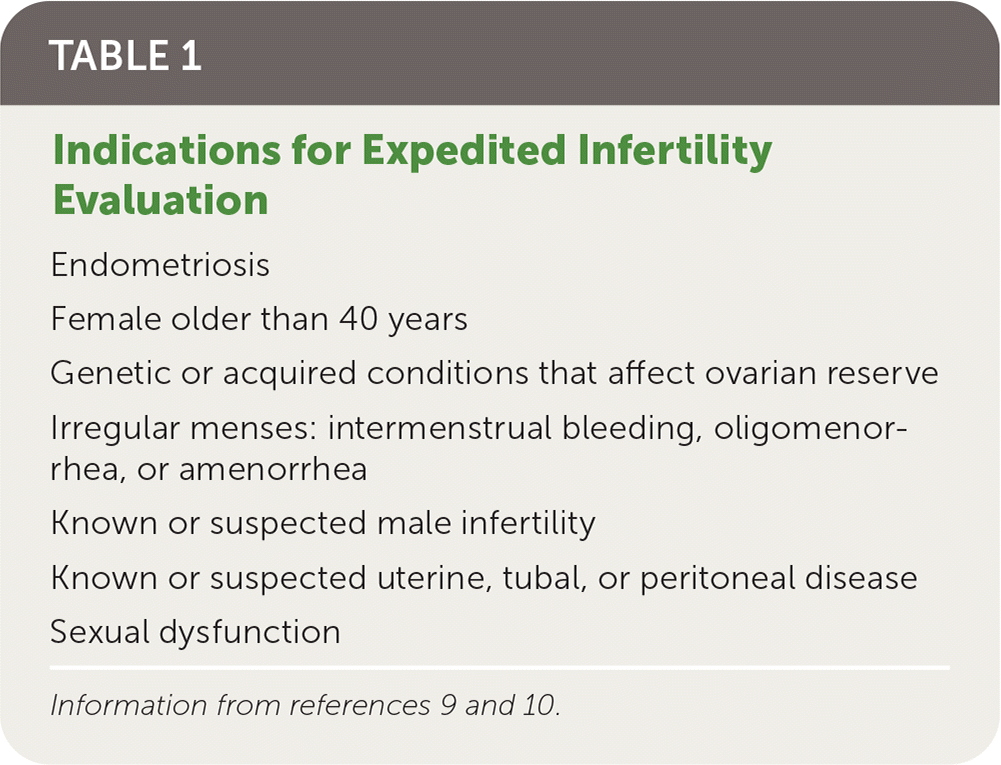
| Endometriosis |
| Female older than 40 years |
| Genetic or acquired conditions that affect ovarian reserve |
| Irregular menses: intermenstrual bleeding, oligomenorrhea, or amenorrhea |
| Known or suspected male infertility |
| Known or suspected uterine, tubal, or peritoneal disease |
| Sexual dysfunction |
Female Factor Infertility
INITIAL EVALUATION
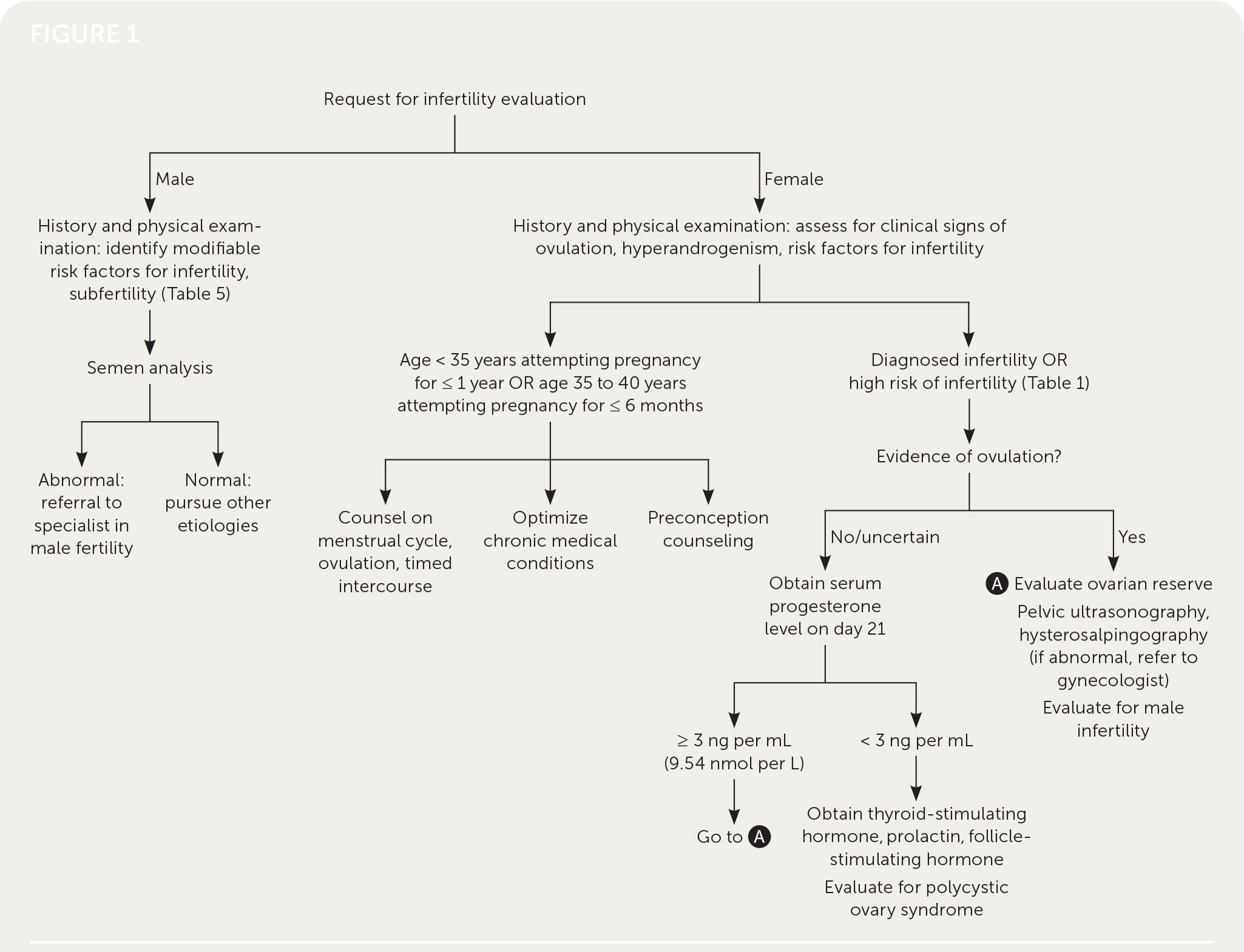
Physicians should obtain a comprehensive medical history, including duration and types of previous infertility treatment; obstetric, menstrual, contraceptive, surgical, and sexual history; a review of systems, medications, allergies, and teratogenic exposures (retinoids, valproate, warfarin, lithium); and a family history. The physical examination should be comprehensive, emphasizing the thyroid, breast, and pelvic areas (Table 2).9,10,13 Pelvic ultrasonography and hysterosalpingography are often performed during the initial workup for infertility; however, the decision to perform imaging should be guided by history and physical examination. Imaging that focuses on pathology or modifiable conditions is more high yield than routine imaging9 (Table 39,10). Figure 1 presents an algorithm for the evaluation of infertility.9–11,14–17
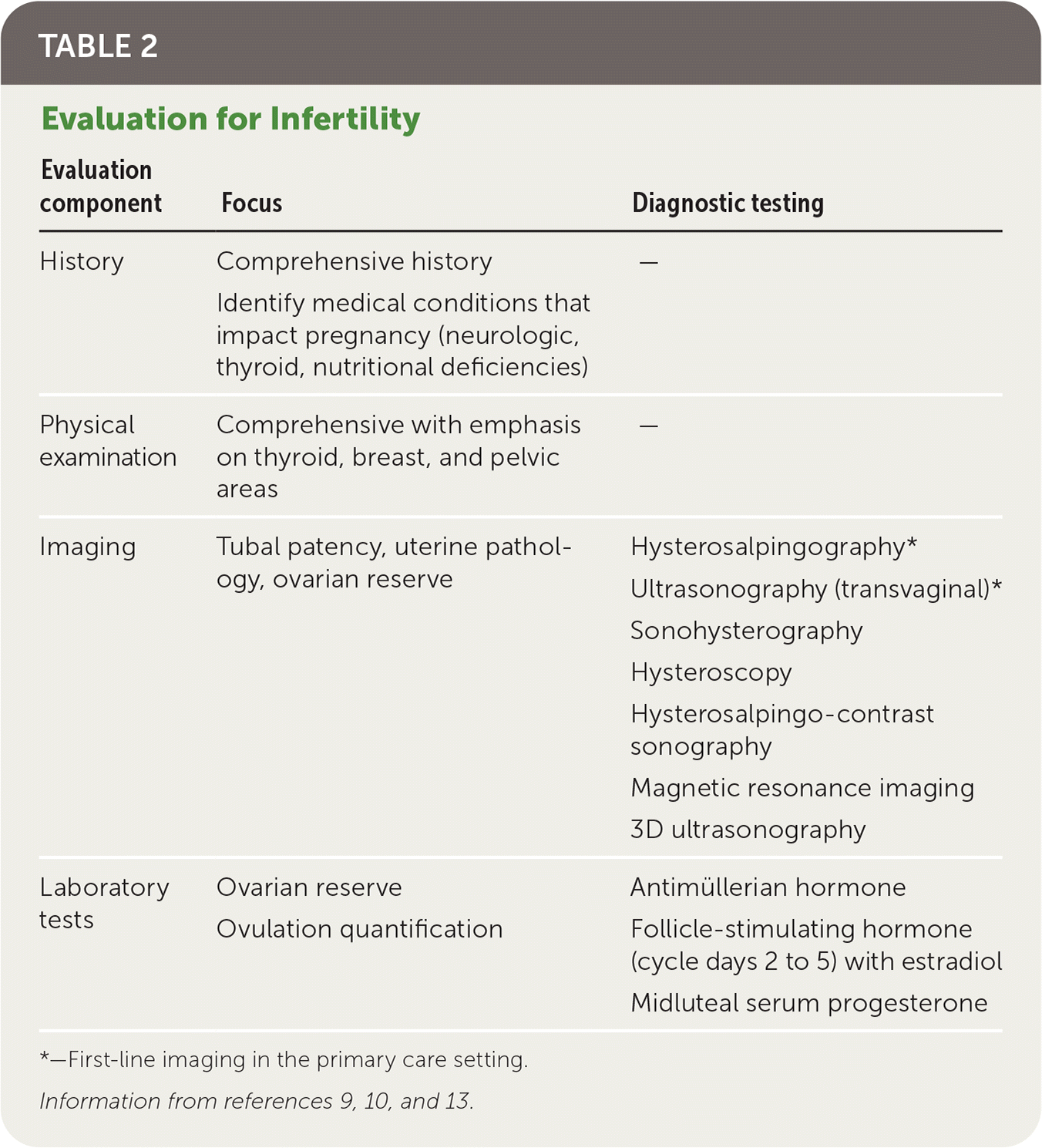
| Evaluation component | Focus | Diagnostic testing |
|---|---|---|
| History | Comprehensive history Identify medical conditions that impact pregnancy (neurologic, thyroid, nutritional deficiencies) | — |
| Physical examination | Comprehensive with emphasis on thyroid, breast, and pelvic areas | — |
| Imaging | Tubal patency, uterine pathology, ovarian reserve | Hysterosalpingography* Ultrasonography (transvaginal)* Sonohysterography Hysteroscopy Hysterosalpingo-contrast sonography Magnetic resonance imaging 3D ultrasonography |
| Laboratory tests | Ovarian reserve Ovulation quantification | Antimüllerian hormone Follicle-stimulating hormone (cycle days 2 to 5) with estradiol Midluteal serum progesterone |
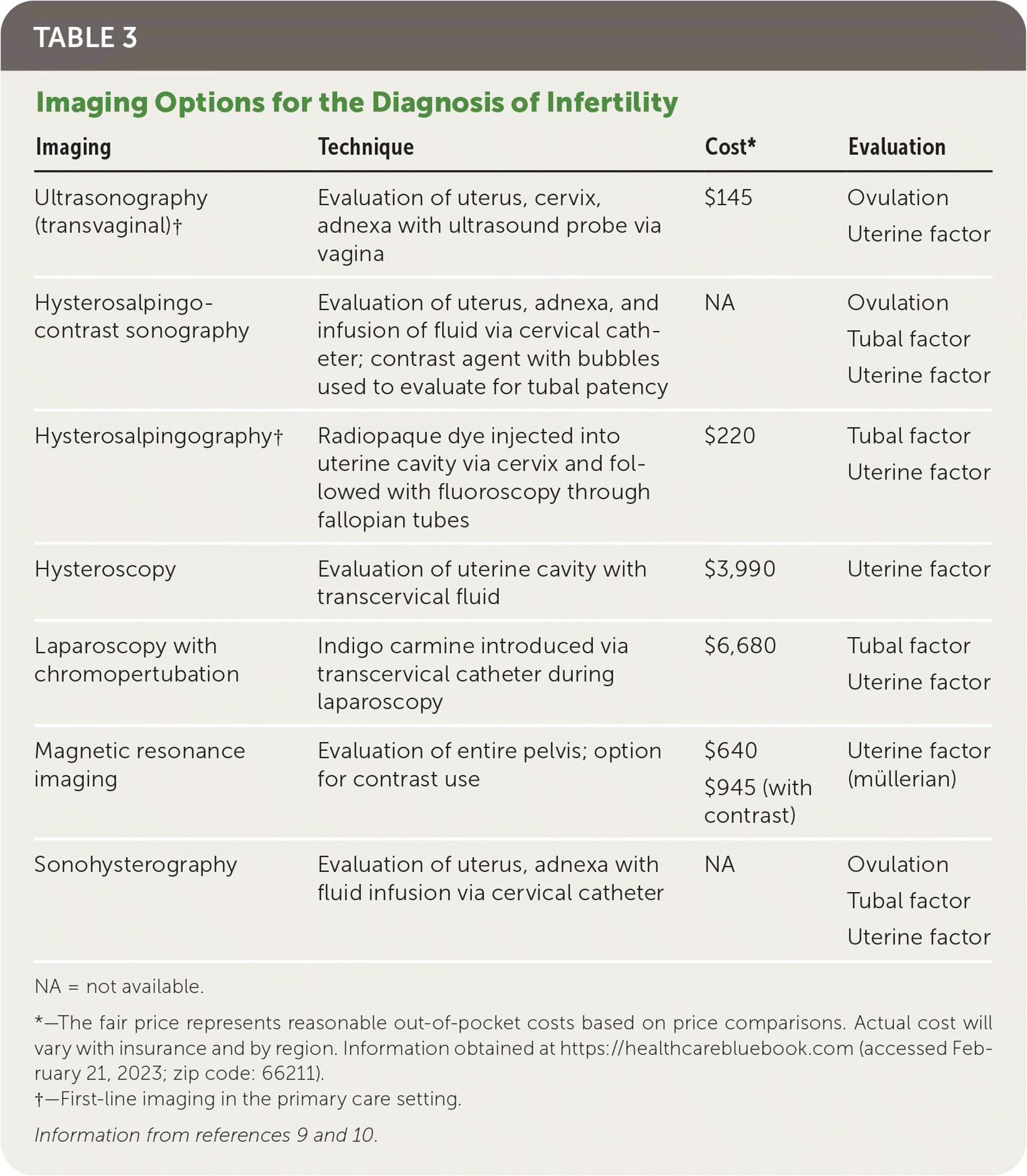
| Imaging | Technique | Cost* | Evaluation |
|---|---|---|---|
| Ultrasonography (transvaginal)† | Evaluation of uterus, cervix, adnexa with ultrasound probe via vagina | $145 | Ovulation Uterine factor |
| Hysterosalpingo-contrast sonography | Evaluation of uterus, adnexa, and infusion of fluid via cervical catheter; contrast agent with bubbles used to evaluate for tubal patency | NA | Ovulation Tubal factor Uterine factor |
| Hysterosalpingography† | Radiopaque dye injected into uterine cavity via cervix and followed with fluoroscopy through fallopian tubes | $220 | Tubal factor Uterine factor |
| Hysteroscopy | Evaluation of uterine cavity with transcervical fluid | $3,990 | Uterine factor |
| Laparoscopy with chromopertubation | Indigo carmine introduced via transcervical catheter during laparoscopy | $6,680 | Tubal factor Uterine factor |
| Magnetic resonance imaging | Evaluation of entire pelvis; option for contrast use | $640 $945 (with contrast) | Uterine factor (müllerian) |
| Sonohysterography | Evaluation of uterus, adnexa with fluid infusion via cervical catheter | NA | Ovulation Tubal factor Uterine factor |
UTERINE FACTOR
Endometrial polyps, leiomyomas, uterine synechiae, and müllerian anomalies can affect fertility. Fibroids occur in up to 70% of females.18 Subserosal fibroids do not appear to affect fertility, but submucosal fibroids can reduce implantation and pregnancy rates.19 Müllerian anomalies can impair implantation and increase the risk of early pregnancy loss. Postsurgical cervical scarring and stenosis and decreased cervical mucus may affect the progression of sperm from the vagina into the uterus. However, evaluation of cervical mucus is no longer routinely used in infertility evaluation.11
TUBAL FACTOR
Tubal factor should be strongly considered when there is a history of sexually transmitted infections, pelvic inflammatory disease, previous abdominal or pelvic surgery, or endometriosis. These conditions may lead to tubal obstruction or impaired tubal motility, which affects the ability to pick up and transmit the oocyte or embryo.8
OVARIAN RESERVE
At the onset of puberty, the ovarian reserve has diminished to less than 10% of the initial embryonic oocyte count (from approximately 7 million to 500,000). These numbers continue decreasing over time through atresia and ovulation.12,20 The measurement of ovarian reserve is the assessment of reproductive potential as a function of the number of oocytes.10 Although variable among females of the same chronologic age, ovarian reserve correlates directly with age and diminishes in all females over time.13
There are multiple indicators of ovarian reserve (Table 4).21 Antimüllerian hormone, follicle-stimulating hormone (FSH), estradiol, and inhibin B are considered biomarkers of ovarian reserve. FSH, estradiol, inhibin B, and antral follicle count are cycle-dependent, whereas antimüllerian hormone is cycle-independent. Although antimüllerian hormone and FSH are indicators of ovarian reserve, they are unreliable markers of fertility in women 30 to 44 years of age without previous infertility.9,13
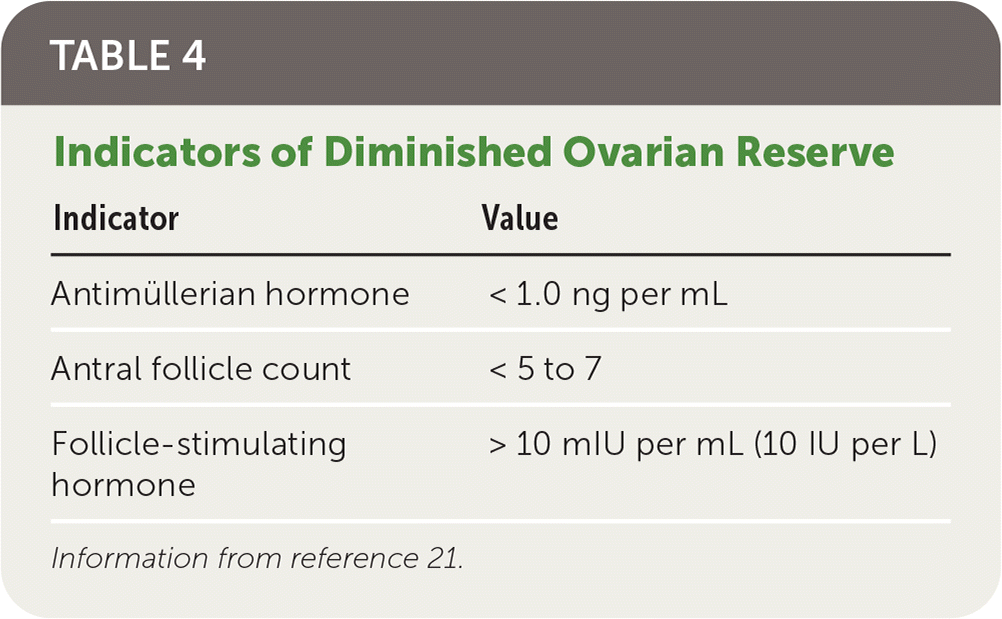
| Indicator | Value |
|---|---|
| Antimüllerian hormone | < 1.0 ng per mL |
| Antral follicle count | < 5 to 7 |
| Follicle-stimulating hormone | > 10 mIU per mL (10 IU per L) |
Primary ovarian insufficiency is the depletion or dysfunction of ovarian follicles with cessation of menses before 40 years of age. Primary ovarian insufficiency may be caused by chemotherapy (alkylating agents) or radiation (greater than 10 gray) and occurs in fragile X syndrome carriers. A person younger than 40 years with an elevated FSH level or family history of early ovarian failure should be tested for fragile X syndrome carrier mutation. Other known causes are endocrinopathies, infiltrative or infectious processes, pelvic surgery, and autoimmune disorders.22
OVULATORY DYSFUNCTION
Ovulatory dysfunction can manifest as oligomenorrhea, amenorrhea, or abnormal uterine bleeding. Polycystic ovary syndrome accounts for most ovulatory infertility, but patients should be evaluated for other causes, including obesity and hypothalamic, pituitary, and thyroid disease. Polycystic ovary syndrome is characterized by hyperandrogenism, oligomenorrhea, or amenorrhea, and polycystic appearance of ovaries on ultrasonography.9 A midluteal phase (day 21) serum progesterone level can be measured to determine ovulatory status. Progesterone levels less than 3 ng per mL (9.54 nmol per L) indicate anovulation.
OTHER CONDITIONS
Obesity affects fertility in females and males. In males, obesity is associated with reduced semen quality and impaired erectile function.23 In females, obesity affects menstrual function, ovulatory function, and oocyte morphology, which may impair fertilization and increase rates of miscarriage and obstetric complications.23,24
Thyroid disease and hyperprolactinemia are other factors that affect ovulation. Hypothyroidism causes a decrease in sex hormone–binding globulin and increased unbound testosterone and estradiol, which alters the hypothalamic-pituitary-ovarian axis by affecting the pulsatile release of gonadotropin-releasing hormone needed for normal follicular development and ovulation. Hyperthyroidism may lead to menstrual disturbances.25 Hyperprolactinemia causes anovulation by inhibiting gonadotropin-releasing hormone secretion.2
Male Factor Infertility
The evaluation of the male partner should include a history, physical examination, and semen analysis.26 The history should focus on reproductive history, presence of sexual dysfunction (e.g., impaired libido, erectile dysfunction), environmental or toxin exposure (heavy metals, pesticides), tobacco and cannabis use, childhood illness (e.g., mumps, cryptorchidism), developmental history, medication use (including exogenous testosterone use), and sexually transmitted infections14 (Table 527). The physical examination should include body mass index and penile and testicular assessment. The absence of the vas deferens on physical examination should prompt cystic fibrosis testing. A semen analysis should be obtained after two to five days of abstinence and is recommended as the first step in the evaluation of infertility in males. Semen should be collected and submitted within one hour of production. If the semen is abnormal, the timing for repeat analysis should be individualized, and referral to a urologist is recommended.15 The World Health Organization laboratory manual provides parameters for normal semen analysis (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8706130/pdf/life-11-01368.pdf). If azoospermia (i.e., no sperm in the ejaculate) is noted on semen analysis, genetic testing for Y chromosome microdeletion should be performed.14

| Alcohol use | Obesity |
| Environmental or occupational exposure to toxic chemicals | Smoking |
| Varicocele | |
| Illicit drug use |
Unexplained Infertility
The diagnosis of unknown or unexplained infertility is made when there is an absence of an identifiable cause for infertility. The evaluation finds normal ovulatory function, a normal semen analysis, and at least one patent fallopian tube. Unexplained infertility may account for 30% of infertility cases.28
Treatment of Infertility
NONGYNECOLOGIC
Optimal treatment of hyperprolactinemia and hypothyroidism, which can manifest as amenorrhea in reproductive-aged women, should result in the resumption of normal menstrual cycles and ovulation. Other concurrent etiologies of infertility can be considered if pregnancy does not occur.
OVARIAN
Methods for ovulation induction include oral medications, clomiphene and letrozole, and exogenous gonadotropins. Clomiphene is a selective estrogen receptor modulator that mimics a hypoestrogenic state, leading to increased FSH and the development of multiple dominant follicles.27 The initial dosage for clomiphene is 50 mg per day for five days, starting on days 2 to 5 of the menstrual cycle, and is increased to 100 mg per day. Letrozole, an aromatase inhibitor, should be used for five days (days 3 to 7 of the menstrual cycle). The typical dosage is 2.5 to 7.5 mg per day. If pregnancy is not achieved after six cycles, the patient should be referred to an infertility specialist.
All ovulation induction medications confer a risk of ovarian hyperstimulation syndrome and multifetal gestation. Ovarian hyperstimulation syndrome manifests as abdominal pain and distention, ascites, gastrointestinal problems, respiratory compromise, oliguria, hemoconcentration, and thromboembolism and is most common with the use of gonadotropins and high-dose oral ovulation induction medications. Ovarian hyperstimulation syndrome is treated with supportive care, including antiemetics, volume replacement, and, in severe cases, paracentesis. Patients should be counseled on these risks before treatment.29
FALLOPIAN TUBES
Occlusion of the fallopian tubes is a major cause of female infertility (25% to 35%).32 Treatment includes tubal cannulation, tubal anastomosis, or in vitro fertilization (IVF). The decision to pursue surgical management vs. assisted reproductive technology (ART) is based on factors related to pregnancy success such as age, ovarian reserve, and location of tubal disease or blockage. Referral to a specialist is warranted for patients with tubal factor infertility.33
UTERUS
Referral to gynecologic surgery is highly recommended when any intrauterine abnormalities are diagnosed. Limited studies suggest that removing submucosal fibroids improves fertility and IVF outcomes. Data are lacking about the mechanisms through which other intrauterine abnormalities affect fertility.34
MALE INFERTILITY
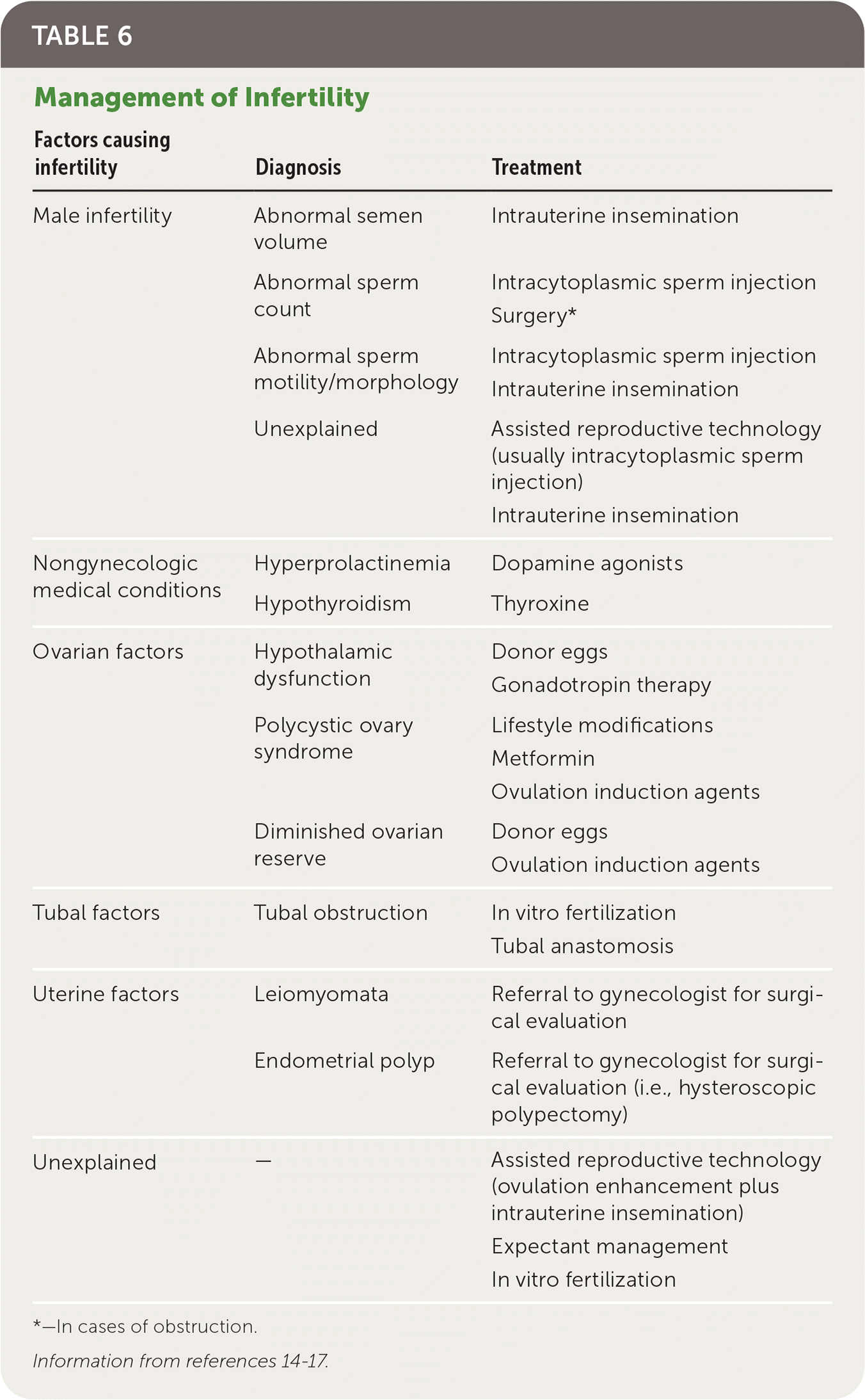
| Factors causing infertility | Diagnosis | Treatment |
|---|---|---|
| Male infertility | Abnormal semen volume | Intrauterine insemination |
| Abnormal sperm count | Intracytoplasmic sperm injection Surgery* | |
| Abnormal sperm motility/morphology | Intracytoplasmic sperm injection Intrauterine insemination | |
| Unexplained | Assisted reproductive technology (usually intracytoplasmic sperm injection) Intrauterine insemination | |
| Nongynecologic medical conditions | Hyperprolactinemia | Dopamine agonists |
| Hypothyroidism | Thyroxine | |
| Ovarian factors | Hypothalamic dysfunction | Donor eggs Gonadotropin therapy |
| Polycystic ovary syndrome | Lifestyle modifications Metformin Ovulation induction agents | |
| Diminished ovarian reserve | Donor eggs Ovulation induction agents | |
| Tubal factors | Tubal obstruction | In vitro fertilization Tubal anastomosis |
| Uterine factors | Leiomyomata | Referral to gynecologist for surgical evaluation |
| Endometrial polyp | Referral to gynecologist for surgical evaluation (i.e., hysteroscopic polypectomy) | |
| Unexplained | — | Assisted reproductive technology (ovulation enhancement plus intrauterine insemination) Expectant management In vitro fertilization |
Azoospermia is commonly caused by obstruction and requires surgical evaluation and correction. The mainstay of treatment for unexplained male factor infertility is ART.17
UNEXPLAINED INFERTILITY
People diagnosed with unexplained infertility should be counseled on expectant management with timed intercourse, lifestyle modifications, and treatment options. Clomiphene or letrozole with intrauterine insemination is recommended as first-line therapy for unexplained infertility and is superior to expectant management (31% vs. 9% live birth rate over three treatment cycles in one randomized controlled trial).17,28 IVF is not recommended as a first-line treatment; however, it should be considered for women 38 years and older.17
LIFESTYLE MODIFICATIONS
In women who are obese with anovulatory cycles, weight loss (5% to 10%) has been shown to improve the rate of spontaneous ovulation and the response to ovulation induction.23
Environmental factors have been identified as having a probable effect on pregnancy rates. The profertility diet (folic acid, vitamins D and B12, fruits, vegetables, and seafood) has been shown to improve pregnancy rates in women undergoing ART.35 A high alcohol intake (greater than two drinks per day), tobacco use, and illicit drug use adversely affect live birth rates. Marijuana use has been shown to decrease sperm count and delay or inhibit ovulation.36
COST AND HEALTH EQUITY ISSUES
Most private insurers pay for the initial infertility evaluation but not the cost of the laboratory tests, imaging, and additional treatment. The median cost of IVF in the United States with medications is $19,200. A total of 70% of women who undergo IVF go into debt.37 Women without insurance coverage for IVF were three times more likely to discontinue treatment after one cycle.38 Mandated IVF insurance is variable and is part of only 17 state-funded programs. New York is the only state where Medicaid is mandated to cover fertility treatment. Medicaid's lack of coverage of fertility services disproportionately impacts women of color.39
This article updates a previous article on this topic by Lindsay and Vitrikas.16
Data Sources: A PubMed search was completed using the key terms female infertility, male infertility, unexplained infertility, treatment, and evaluation. The search included meta-analyses, randomized controlled trials, editorials, clinical trials, and systematic reviews. Also searched were the Centers for Disease Control and Prevention’s National Survey of Family Growth and National Center for Health Statistics, Kaiser Family Foundation’s women’s health policy, and the Society for Assisted Reproductive Technology data. Search dates: March 24, 2022; April 21, 2022; April 28, 2022; May 12, 2022; and March 23, 2023.