
Am Fam Physician. 2023;108(1):online
Author disclosure: No relevant financial relationships.

Details for This Review
Study Population: Children younger than 24 months diagnosed with mild to moderate bronchiolitis in emergency department, inpatient, and outpatient settings
Efficacy End Points: The rate and length of hospitalization, clinical severity score, and readmission rates
Harm End Points: Adverse events (e.g., worsening cough, agitation, bronchospasm, bradycardia, desaturation, vomiting, diarrhea)
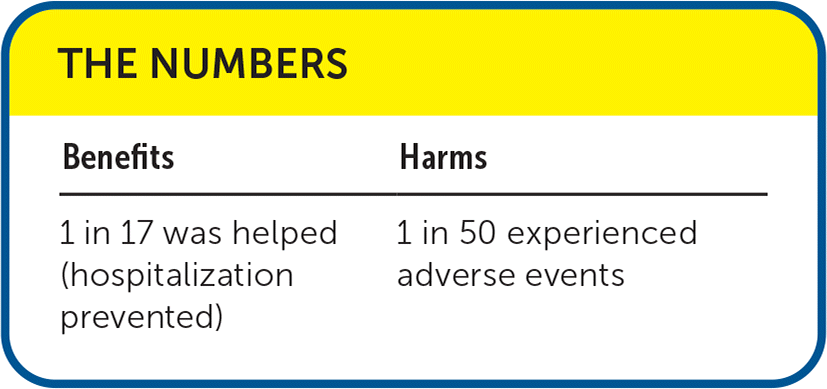
| Benefits | Harms |
|---|---|
| 1 in 17 was helped (hospitalization prevented) | 1 in 50 experienced adverse events |
Narrative: Acute bronchiolitis is the most common lower respiratory tract infection in children younger than two years. A respiratory syncytial virus is responsible for most cases, with almost all infections occurring before two years of age. Approximately 1% to 2% of patients who are infected require hospitalization. Acute bronchiolitis includes peribronchial inflammation, airway edema, and excess mucus secretion that can lead to airway obstruction, atelectasis, and impaired gas exchange. The standard treatment involves supportive therapy with humidified oxygen, fluid intake, mucus clearance, and nutritional supplementation, although additional interventions to reduce airway swelling and mucus plugging are being studied. Nebulized hypertonic saline has been used to lower the viscosity of secretions, improve mucus clearance, and decrease the length of hospitalization among infants and children with bronchiolitis.
The systematic review included 34 randomized controlled trials or quasi-randomized controlled trials (n = 5,205) evaluating the effectiveness and safety of nebulized hypertonic saline (greater than 3%) compared with normal nebulized saline (0.9%) in the treatment of children with bronchiolitis.1 Nebulized hypertonic saline decreased the rate of hospitalization (relative risk = 0.87; 95% CI, 0.78 to 0.97; absolute risk difference [ARD] = 6%; number needed to treat = 17; eight trials; 1,760 patients; low-certainty evidence) compared with normal nebulized saline. In patients who were hospitalized, nebulized hypertonic saline treatments decreased the length of hospital stay compared with normal nebulized saline treatments (mean difference = −0.40 days; 95% CI, −0.69 to −0.11; 21 trials; 2,479 infants; low-certainty evidence). Postinhalation severity scores improved slightly following hypertonic saline administration, averaging 11%, 21%, and 22% lower on days 1, 2, and 3, respectively. The rate of readmission at 28 days did not differ between the groups.
Safety data were reported for 27 trials (n = 4,416). However, differences in reporting prevented meta-analysis of this information. Using the data from individual studies, 88 out of 2,213 children in the treatment group and 43 out of 2,135 in the control group experienced adverse events (ARD = 2%; number needed to harm = 50). Most adverse events (e.g., vomiting, diarrhea, bradycardia, increased coughing) were mild and resolved spontaneously.
Caveats: The certainty of evidence is rated low to very low. A high degree of statistical and clinical heterogeneity was attributed to differences in treatment regimens, clinical definitions of bronchiolitis, and disease severity. Most studies used 0.9% nebulized saline as a comparison to allow the studies to be double blinded. However, because normal saline inhalation could also provide physiologic benefits in bronchiolitis, it is not technically a placebo and could have decreased the effect size of hypertonic saline. Some studies included a bronchodilator, which could also affect the results. Several trials were completed but never published or reported on clinical trial registries, likely contributing to publication bias. In addition, two out of eight trials contributed 73% of the weight of the effects in evaluating the rate of hospitalization. This review incorporated studies from multiple countries, which could have resulted in different standards of care for hospitalization and discharge criteria.
Conclusion: The existing low-certainty data suggest that the benefits of nebulized hypertonic saline administration for the treatment of bronchiolitis outweigh the potential harms. With most adverse events resolving spontaneously, it can be reasonably assumed that nebulized hypertonic saline is a safe treatment in children with bronchiolitis. The use of hypertonic saline in the treatment of bronchiolitis may decrease the rate of hospitalization and shorten the length of hospitalization. We have assigned a color recommendation of yellow (unclear benefits) for nebulized hypertonic saline for the treatment of infants and children with bronchiolitis because of the noted limitations of the data.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army Medical Corps, the U.S. Army, or the U.S. Department of Defense.
