
Am Fam Physician. 2023;108(1):100-104
Author disclosure: No relevant financial relationships.
Key Points for Practice
• Bisphosphonates, for up to five years orally or three years intravenously, are first-line therapy for osteoporosis.
• Denosumab injections every six months improve bone density more quickly than bisphosphonates, although bone density improvements fade within months after discontinuation unless bisphosphonates are started.
• Parathyroid hormone analog therapy for up to two years dramatically improves bone density and reduces fractures but requires subsequent bisphosphonate use to maintain benefit.
• One year of treatment with romosozumab, a sclerostin-binding analog, followed by one year of alendronate reduces fracture risk more than two years of alendronate therapy alone.
From the AFP Editors
More than two-thirds of osteoporotic fractures occur in women, and one-half of postmenopausal women will experience an osteoporotic fracture. The American College of Obstetricians and Gynecologists (ACOG) has published new recommendations for managing this undertreated condition, including guidance on new medications and targeted treatments.
Health Inequities in Osteoporosis
Despite an increased risk of subsequent fracture within the first two years following a fracture, only one-fourth of women 60 years and older receive osteoporosis treatment during the first year. Black women are less likely than White women to receive treatment after diagnosis of osteoporosis, even after adjusting for insurance and socioeconomic status. Black women also have higher mortality in the year following a major fragility fracture.
Diagnosis
Although osteoporosis can be diagnosed clinically after a fragility fracture from a fall of less than standing height, dual-energy x-ray absorptiometry is the preferred means of identifying bone loss before a fracture occurs. Dual-energy x-ray absorptiometry results are reported as a T-score of bone density referenced to healthy young women, and osteoporosis is identified at a T-score of −2.5.
Secondary Causes of Bone Loss
Osteoporosis can stem from secondary causes (Table 1). Although there is no direct evidence of benefit in patients with one or more of these factors or in patients with very low bone mineral density or a history of multiple or recent fractures, it may be appropriate to obtain a complete blood count, complete metabolic panel, thyroid-stimulating hormone level with or without free thyroxine, 25-hydroxyvitamin D level, and 24-hour urine collection for calcium, sodium, and creatinine excretion.
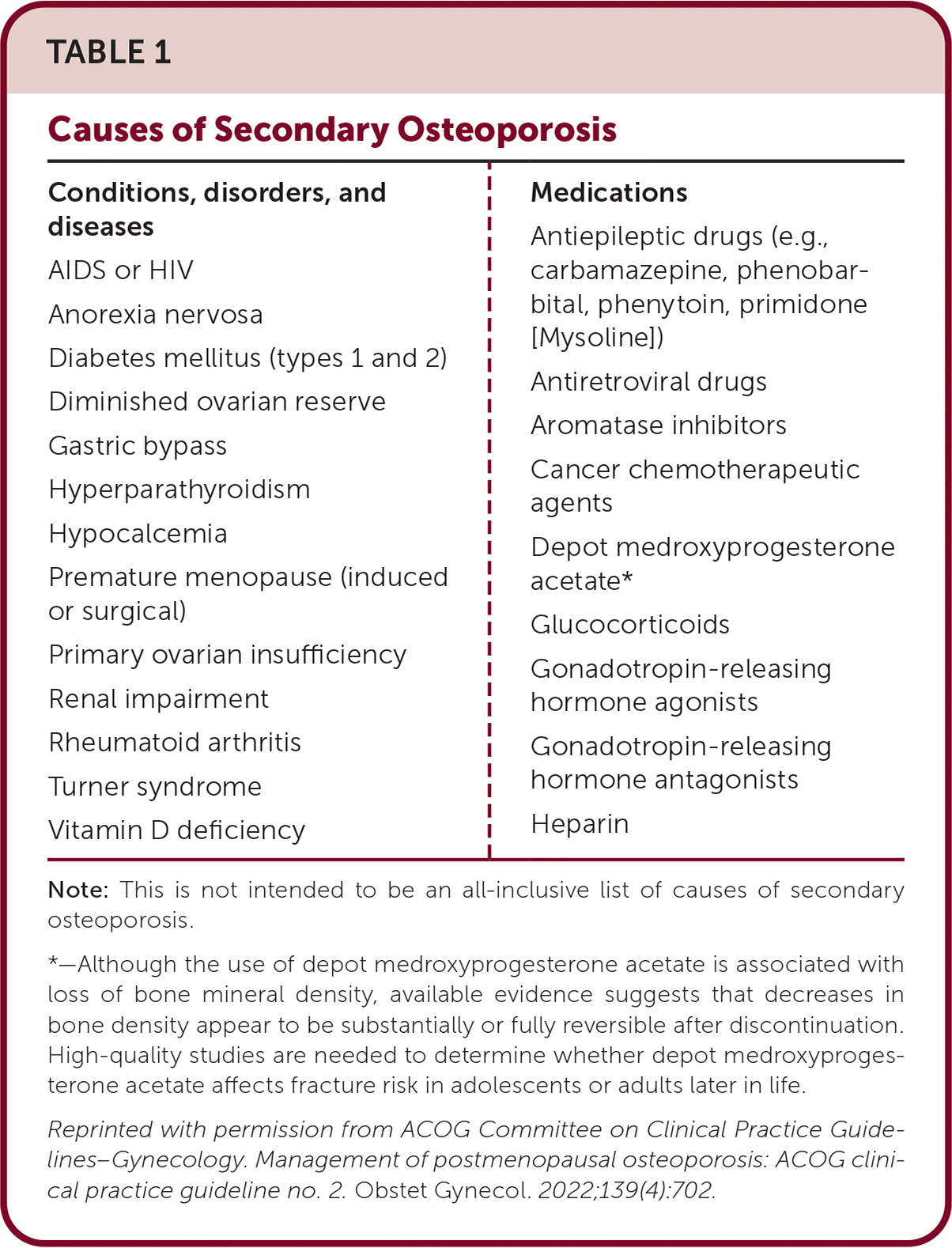
| Conditions, disorders, and diseases AIDS or HIV Anorexia nervosa Diabetes mellitus (types 1 and 2) Diminished ovarian reserve Gastric bypass Hyperparathyroidism Hypocalcemia Premature menopause (induced or surgical) Primary ovarian insufficiency Renal impairment Rheumatoid arthritis Turner syndrome Vitamin D deficiency | Medications Antiepileptic drugs (e.g., carbamazepine, phenobarbital, phenytoin, primidone [Mysoline]) Antiretroviral drugs Aromatase inhibitors Cancer chemotherapeutic agents Depot medroxyprogesterone acetate* Glucocorticoids Gonadotropin-releasing hormone agonists Gonadotropin-releasing hormone antagonists Heparin |
Indications for Medical Treatment
Medication is recommended for women with postmenopausal osteoporosis who have any of the following:
T-score of −2.5 or less for hip, lumbar spine, femoral neck, or distal third of radius
History of fragility fracture, including incidental or asymptomatic vertebral fracture
T-score between −1.0 and −2.5 and increased risk of fracture based on results from a clinical risk assessment tool, such as the Fracture Risk Assessment Tool (https://www.sheffield.ac.uk/FRAX/index.aspx)
Antiresorptive Agents
Antiresorptive agents decrease resorption of bone to increase bone density and are recommended for fracture prevention in most cases of osteoporosis.
BISPHOSPHONATES
Bisphosphonates inhibit osteoclast bone resorption and are the preferred initial therapy for osteoporosis. All bisphosphonates reduce vertebral fractures and most also reduce nonvertebral fractures (Table 2). Common adverse effects include musculoskeletal aches and pains, gastrointestinal irritation, esophageal reflux, and ulceration. Rare adverse effects include osteonecrosis of the jaw, atypical femoral fracture, and esophageal cancer.
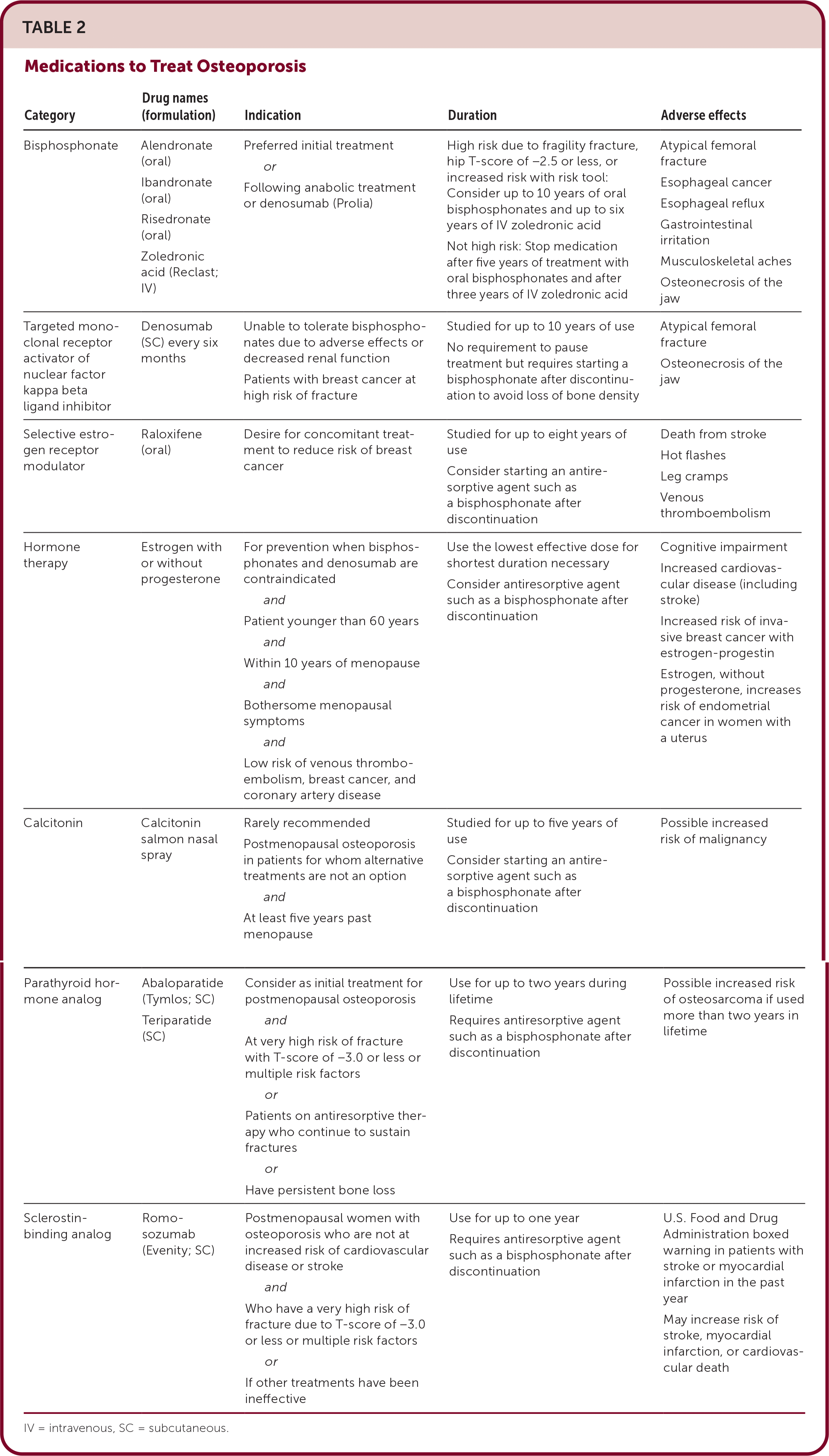
| Category | Drug names (formulation) | Indication | Duration | Adverse effects |
|---|---|---|---|---|
| Bisphosphonate | Alendronate (oral) Ibandronate (oral) Risedronate (oral) Zoledronic acid (Reclast; IV) | Preferred initial treatment or Following anabolic treatment or denosumab (Prolia) | High risk due to fragility fracture, hip T-score of −2.5 or less, or increased risk with risk tool: Consider up to 10 years of oral bisphosphonates and up to six years of IV zoledronic acid Not high risk: Stop medication after five years of treatment with oral bisphosphonates and after three years of IV zoledronic acid | Atypical femoral fracture Esophageal cancer Esophageal reflux Gastrointestinal irritation Musculoskeletal aches Osteonecrosis of the jaw |
| Targeted monoclonal receptor activator of nuclear factor kappa beta ligand inhibitor | Denosumab (SC) every six months | Unable to tolerate bisphosphonates due to adverse effects or decreased renal function Patients with breast cancer at high risk of fracture | Studied for up to 10 years of use No requirement to pause treatment but requires starting a bisphosphonate after discontinuation to avoid loss of bone density | Atypical femoral fracture Osteonecrosis of the jaw |
| Selective estrogen receptor modulator | Raloxifene (oral) | Desire for concomitant treatment to reduce risk of breast cancer | Studied for up to eight years of use Consider starting an antiresorptive agent such as a bisphosphonate after discontinuation | Death from stroke Hot flashes Leg cramps Venous thromboembolism |
| Hormone therapy | Estrogen with or without progesterone | For prevention when bisphosphonates and denosumab are contraindicated and Patient younger than 60 years and Within 10 years of menopause and Bothersome menopausal symptoms and Low risk of venous thromboembolism, breast cancer, and coronary artery disease | Use the lowest effective dose for shortest duration necessary Consider antiresorptive agent such as a bisphosphonate after discontinuation | Cognitive impairment Increased cardiovascular disease (including stroke) Increased risk of invasive breast cancer with estrogen-progestin Estrogen, without progesterone, increases risk of endometrial cancer in women with a uterus |
| Calcitonin | Calcitonin salmon nasal spray | Rarely recommended Postmenopausal osteoporosis in patients for whom alternative treatments are not an option and At least five years past menopause | Studied for up to five years of use Consider starting an antiresorptive agent such as a bisphosphonate after discontinuation | Possible increased risk of malignancy |
| Parathyroid hormone analog | Abaloparatide (Tymlos; SC) Teriparatide (SC) | Consider as initial treatment for postmenopausal osteoporosis and At very high risk of fracture with T-score of −3.0 or less or multiple risk factors or Patients on antiresorptive therapy who continue to sustain fractures or Have persistent bone loss | Use for up to two years during lifetime Requires antiresorptive agent such as a bisphosphonate after discontinuation | Possible increased risk of osteosarcoma if used more than two years in lifetime |
| Sclerostin-binding analog | Romosozumab (Evenity; SC) | Postmenopausal women with osteoporosis who are not at increased risk of cardiovascular disease or stroke and Who have a very high risk of fracture due to T-score of −3.0 or less or multiple risk factors or If other treatments have been ineffective | Use for up to one year Requires antiresorptive agent such as a bisphosphonate after discontinuation | U.S. Food and Drug Administration boxed warning in patients with stroke or myocardial infarction in the past year May increase risk of stroke, myocardial infarction, or cardiovascular death |
Oral bisphosphonates are poorly absorbed and must be taken on an empty stomach at least 30 minutes before eating. The patient should remain upright for 30 minutes to avoid esophageal irritation. Oral bisphosphonates are contraindicated in patients with hypocalcemia, esophageal disorders, and gastrointestinal malabsorption (e.g., gastric bypass), but intravenous bisphosphonates can generally be used. All bisphosphonates are contraindicated in patients with chronic kidney disease that has progressed to an estimated glomerular filtration rate of 35 mL per minute or less.
Because prolonged use of bisphosphonates is associated with increased risk of femoral fracture and possibly osteonecrosis of the jaw, stopping after five years of oral treatment or three years of intravenous treatment is recommended. Limited evidence suggests that treatment beyond these time frames does not reduce the likelihood of nonvertebral fractures. In patients at high risk of fracture due to previous osteoporotic fracture or a hip T-score of −2.5 or lower, or with increased risk based on risk assessment tool results, longer initial treatment of up to 10 years for oral bisphosphonates and up to six years for intravenous zoledronic acid (Reclast) is suggested. Duration of drug holidays is unclear; however, expert opinion recommends two to four years. Therapy can later be restarted if the risk of fracture increases.
DENOSUMAB
Denosumab (Prolia) is a human monoclonal antibody that inhibits the receptor activator of nuclear factor kappa beta ligand to reduce osteoclast activity. The recommendations suggest offering denosumab to patients who prefer an injection every six months over oral therapy. It can also be used in patients with chronic kidney disease or patients with breast cancer treated with aromatase inhibitors. Like bisphosphonates, denosumab rarely can lead to osteonecrosis of the jaw and atypical femoral fractures and is contraindicated in people with a history of either.
A bisphosphonate medication should be started if denosumab treatment is stopped because bone density fades within months, increasing fracture risk unless another agent is started.
SELECTIVE ESTROGEN RECEPTOR MODULATORS
Raloxifene acts as an estrogen agonist in bone, reducing bone turnover and resorption. The guidelines suggest raloxifene only for postmenopausal patients at increased risk of vertebral fracture and breast cancer who are at low risk of venous thromboembolism and do not have significant vasomotor symptoms. It has not been demonstrated to reduce hip or other nonvertebral fracture rates. Common adverse effects include leg cramps and hot flashes, and raloxifene use slightly increases the risk of venous thromboembolism and stroke. Raloxifene is contraindicated with current or previous venous thromboembolism and should be avoided in patients with hepatic impairment.
HORMONE THERAPIES
Estrogen, with or without progesterone, increases bone density and treats vasomotor symptoms in postmenopausal women. However, the guidelines recommend estrogen only for prevention of fracture in select patients.
A combination of conjugated estrogen and the selective estrogen receptor modulator bazedoxifene (Duavee) can reduce vasomotor symptoms in menopause while slightly improving bone density. The impact on fracture risk is unknown.
Calcitonin salmon nasal spray is rarely used because of an increased risk of malignancy and the availability of more effective therapies. Calcitonin is indicated for women who are at least five years past menopause and cannot use other treatments.
Anabolic Agents
Anabolic agents restore lost bone structure in advanced osteoporosis and can be considered in patients with a very high risk of fracture. They should be followed by an antiresorptive agent to preserve bone density improvements.
PARATHYROID HORMONE ANALOGS
In patients with very high risk of fracture due to a history of severe or multiple vertebral fractures, a T-score of −3 or lower, or multiple risk factors, the parathyroid hormone analogs teriparatide and abaloparatide (Tymlos) are highly effective. Parathyroid hormone analogs can also be used in patients whose symptoms do not respond to antiresorptive therapy. Unlike antiresorptive agents, they can restore bone mass that is already lost in patients with advanced osteoporosis. Treatment duration should be limited to two years because of a possible risk of osteosarcoma.
Although teriparatide and abaloparatide improve bone density and reduce vertebral and nonvertebral fracture, abaloparatide increases bone density more than teriparatide and more effectively reduces new vertebral fracture.
SCLEROSTIN-BINDING ANALOGS
Like the parathyroid hormone analogs, the sclerostin-binding analog romosozumab (Evenity) is an anabolic agent for patients at very high risk of fracture. Use of romosozumab is limited to one year based on the limited duration of studies to date. Treating with romosozumab for one year followed by one year of alendronate reduces fracture risk compared with alendronate treatment for 24 months. Romosozumab use increases the risk of myocardial infarction, stroke, and cardiovascular death, and there is a U.S. Food and Drug Administration boxed warning against use in patients with myocardial infarction or stroke within one year. Romosozumab is contraindicated in patients with hypocalcemia and rarely can lead to osteonecrosis of the jaw and atypical femoral fracture.
Nonpharmacologic Interventions
LIFESTYLE INTERVENTIONS
Fall prevention is important to prevent fractures and should accompany pharmacologic treatments. Routine moderate- to high-impact exercise reduces falls and prevents bone loss.
CALCIUM AND VITAMIN D
Whereas osteoporosis studies nearly always include calcium and vitamin D supplementation, a review by the U.S. Preventive Services Task Force found no benefit from supplementation in average-risk adults. In postmenopausal women, dietary calcium intake of 1,000 to 1,200 mg daily with vitamin D intake of 800 international units daily appears to reduce hip fracture but not vertebral fracture.
COMPLEMENTARY TREATMENTS
Soy isoflavones slightly increase bone density. Flax seeds, green tea extract, and the herbal treatment fufang are not beneficial.
REFERRAL
Referral to an endocrinologist or osteoporosis specialist is suggested for patients with a T-score less than −3.0, new fragility fracture, fragility fracture with normal bone mineral density, osteoporosis not responding to treatment, an endocrine or metabolic cause of secondary osteoporosis, or comorbidities that complicate treatment.
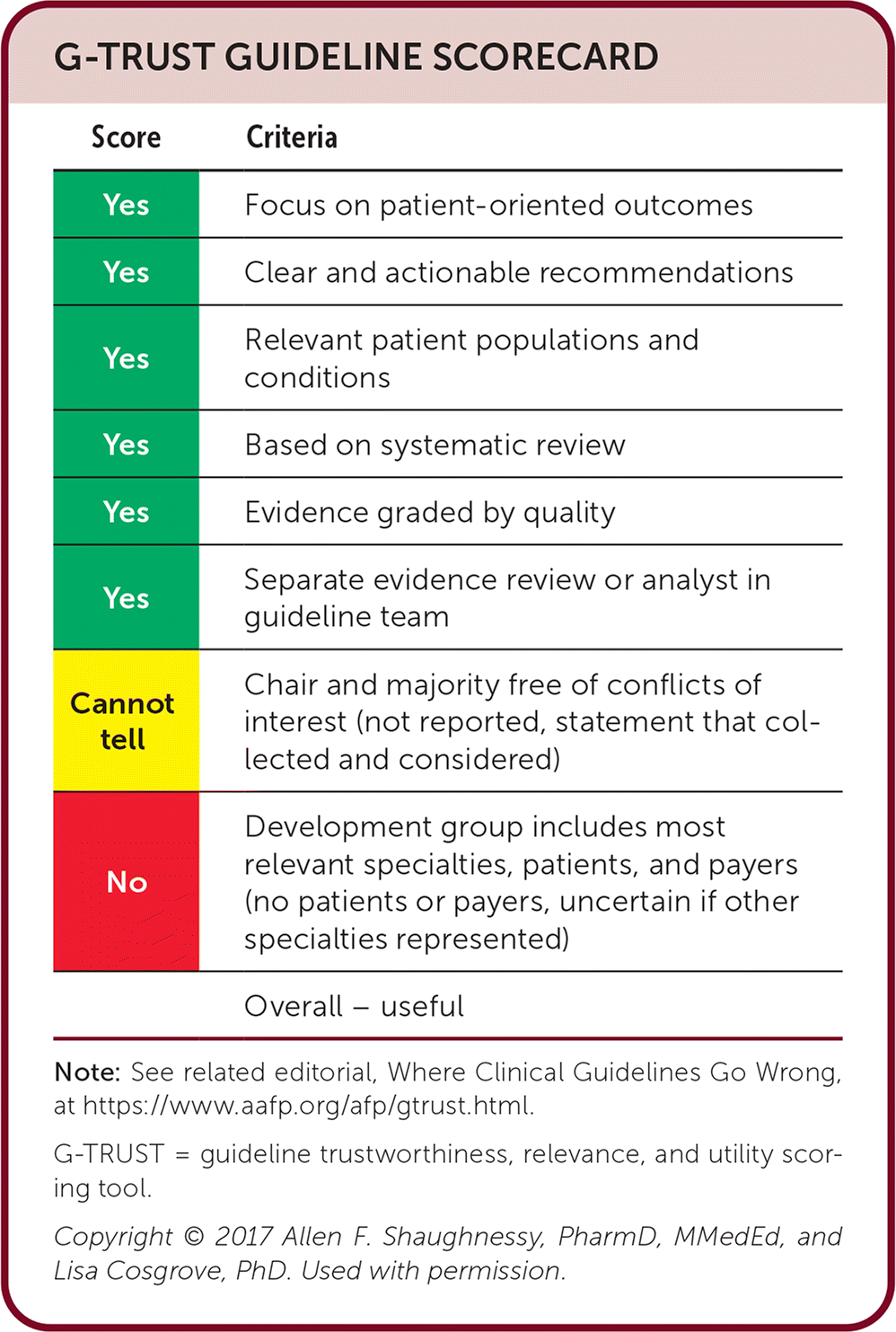
| Score | Criteria |
|---|---|
| Yes | Focus on patient-oriented outcomes |
| Yes | Clear and actionable recommendations |
| Yes | Relevant patient populations and conditions |
| Yes | Based on systematic review |
| Yes | Evidence graded by quality |
| Yes | Separate evidence review or analyst in guideline team |
| Cannot tell | Chair and majority free of conflicts of interest (not reported, statement that collected and considered) |
| No | Development group includes most relevant specialties, patients, and payers (no patients or payers, uncertain if other specialties represented) |
| Overall – useful |
Editor's Note: This guideline is the second true clinical practice guideline from ACOG and the second covering osteoporosis. I find this guideline useful because it is written for the primary care physician and not the endocrinologist. Although the guideline discusses anabolic agents that we are unlikely to prescribe, the information about candidates, risks, and benefits is important before referral to an endocrinologist. The recommendations of this guideline are consistent with the recent AFP article covering osteoporosis (https://www.aafp.org/pubs/afp/issues/2023/0300/osteoporosis.html).—Michael J. Arnold, MD, Assistant Medical Editor
Guideline source: American College of Obstetricians and Gynecologists
Published source: Obstet Gynecol. September 2022;139(4):698–717 [published correction appears in Obstet Gynecol. 2022;140(1):138].
