
NOTE: On July 17, 2023, the U.S. Food and Drug Administration (FDA) approved nirsevimab for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease in neonates and infants born during or entering their first RSV season and in children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season. This article has been revised to reflect the FDA approval. For more information, see the FDA news release.
Am Fam Physician. 2023;108(1):52-57
Patient information: See related handout on respiratory syncytial virus infection.
Author disclosure: No relevant financial relationships.
Bronchiolitis is the most common lower respiratory tract infection in young children. Respiratory syncytial virus (RSV) is the most common viral cause of bronchiolitis. RSV is spread through respiratory droplets, and the number of cases varies with season. For most patients, standard precautions (e.g., hand hygiene, surface cleaning, avoiding contact with sick individuals) are recommended. However, prophylaxis with palivizumab may be considered for infants at high risk. Initial symptoms occur after an incubation period of four to six days and include rhinorrhea, congestion, sneezing, and fever. Signs of lower respiratory tract involvement may follow and include cough, tachypnea, retractions, difficulty feeding, and accessory muscle use. Diagnosis is typically clinical; routine use of radiography or viral testing is not recommended. Treatment of RSV bronchiolitis is mainly supportive. Oxygen saturation should be maintained above 90%. Hydration and nutrition should be maintained by nasogastric or intravenous routes, if needed. Therapies such as bronchodilators, epinephrine, nebulized hypertonic saline, corticosteroids, antibiotics, and chest physiotherapy are not recommended. Although most episodes of RSV bronchiolitis are self-limited, some children have an increased risk of asthma later in life.
Bronchiolitis is the most common lower respiratory tract infection in children younger than five years. Respiratory syncytial virus (RSV) is the most common viral cause of bronchiolitis worldwide. This article summarizes the best available evidence for the management of RSV bronchiolitis.
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| Palivizumab can be considered for RSV prophylaxis in children with increased risk of severe infection.1,10,11 | A | Consensus guidelines, systematic review |
| Radiography and viral testing should not be routinely used in the diagnosis of uncomplicated RSV bronchiolitis.1,22,23,26 | B | Consensus guidelines, observational studies |
| Bronchodilators, prolonged epinephrine use, corticosteroids, and chest physiotherapy should not be used for treatment of RSV bronchiolitis.1,34–38,42,43 | B | Consensus guidelines, systematic reviews, randomized controlled trials |
| Antibiotics should not be used for treatment of RSV bronchiolitis unless there is evidence of a concurrent bacterial infection.1,41 | A | Consensus guidelines, systematic reviews |
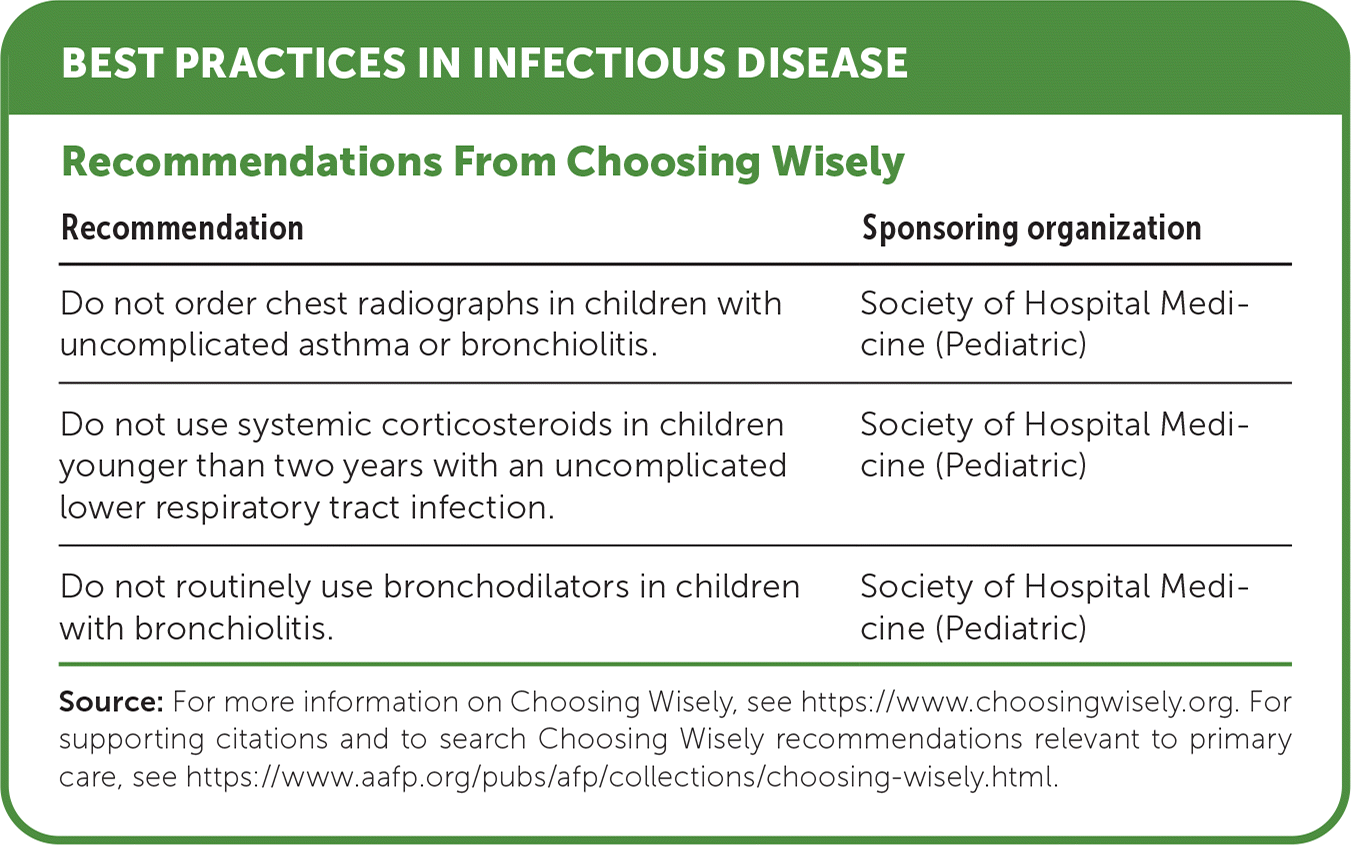
| Recommendation | Sponsoring organization |
|---|---|
| Do not order chest radiographs in children with uncomplicated asthma or bronchiolitis. | Society of Hospital Medicine (Pediatric) |
| Do not use systemic corticosteroids in children younger than two years with an uncomplicated lower respiratory tract infection. | Society of Hospital Medicine (Pediatric) |
| Do not routinely use bronchodilators in children with bronchiolitis. | Society of Hospital Medicine (Pediatric) |
Epidemiology
RSV is spread through respiratory droplets, with symptoms developing after an incubation period of four to six days.1,2
The global incidence of RSV bronchiolitis is estimated to be 8.1 cases per 1,000 children per year, leading to 33 million cases and 3.6 million hospitalizations in 2019.3 In the United States, the incidence of RSV-related hospitalizations in children younger than five years is estimated at 2.9 cases per 1,000 per year.4
The incidence of RSV bronchiolitis varies with region and season. Before the COVID-19 pandemic, cases peaked between mid-October and May in the United States. Since COVID-19 restrictions were lifted, RSV peaks have been delayed to spring and summer.1,5,6 The Centers for Disease Control and Prevention performs surveillance for RSV and reports weekly trends by region.7
Age is the most important risk factor for severe disease and hospitalization. The rate of RSV- related hospitalization in infants younger than one month is estimated to be 25.1 per 1,000 (95% CI, 21.1 to 29.3). Infants born before 36 weeks of gestation are significantly more likely to need hospitalization compared with term infants.1,4
RSV is isolated in up to 83% of RSV cases. Viral coinfection occurs in up to 55% of cases, with human rhinovirus being the most common coisolate.8 A 2017 study over 12 epidemic seasons found that coinfection did not affect disease severity or outcomes.9
Prevention
Standard precautions against the spread of respiratory droplets, such as hand hygiene, surface cleaning, and avoiding contact with sick individuals, are recommended.1
Prophylactic palivizumab, an intramuscular monoclonal antibody, may be considered for children at risk of severe infection (Table 11,2,10). Palivizumab is given monthly during RSV season, for up to five doses (15 mg per kg per dose). It should be discontinued if the child is subsequently hospitalized for RSV.1,10,11
A 2021 Cochrane review demonstrated that palivizumab leads to significantly fewer RSV hospitalizations (number needed to treat [NNT] = 18), but with no significant mortality benefit.11
One dose of nirsevimab at the start of RSV season has been shown decrease the need for outpatient evaluation (NNT = 14) and hospitalization (NNT = 30) in preterm infants (less than 35 weeks).12 In healthy late preterm infants (35 to 37 weeks) and full-term infants, one dose of nirsevimab prevents outpatient visits (NNT = 26) and may prevent hospitalization; however, further study is needed.13 Nirsevimab has been approved by the U.S. Food and Drug Administration (FDA) and is expected to be available at the start of the fall 2023 RSV season.
A recent phase 3 trial has shown that a single intramuscular dose of a bivalent RSV prefusion F vaccine given to pregnant patients between 24 and 36 weeks of gestation is 81.8% (99.5% CI, 40.6 to 96.3) effective in preventing medical evaluation for severe RSV in newborns through 90 days of life. The vaccine is 69.4% (97.59% CI, 44.3 to 84.1) effective through 180 days of life.14 The vaccine has been FDA-approved for high-risk children.
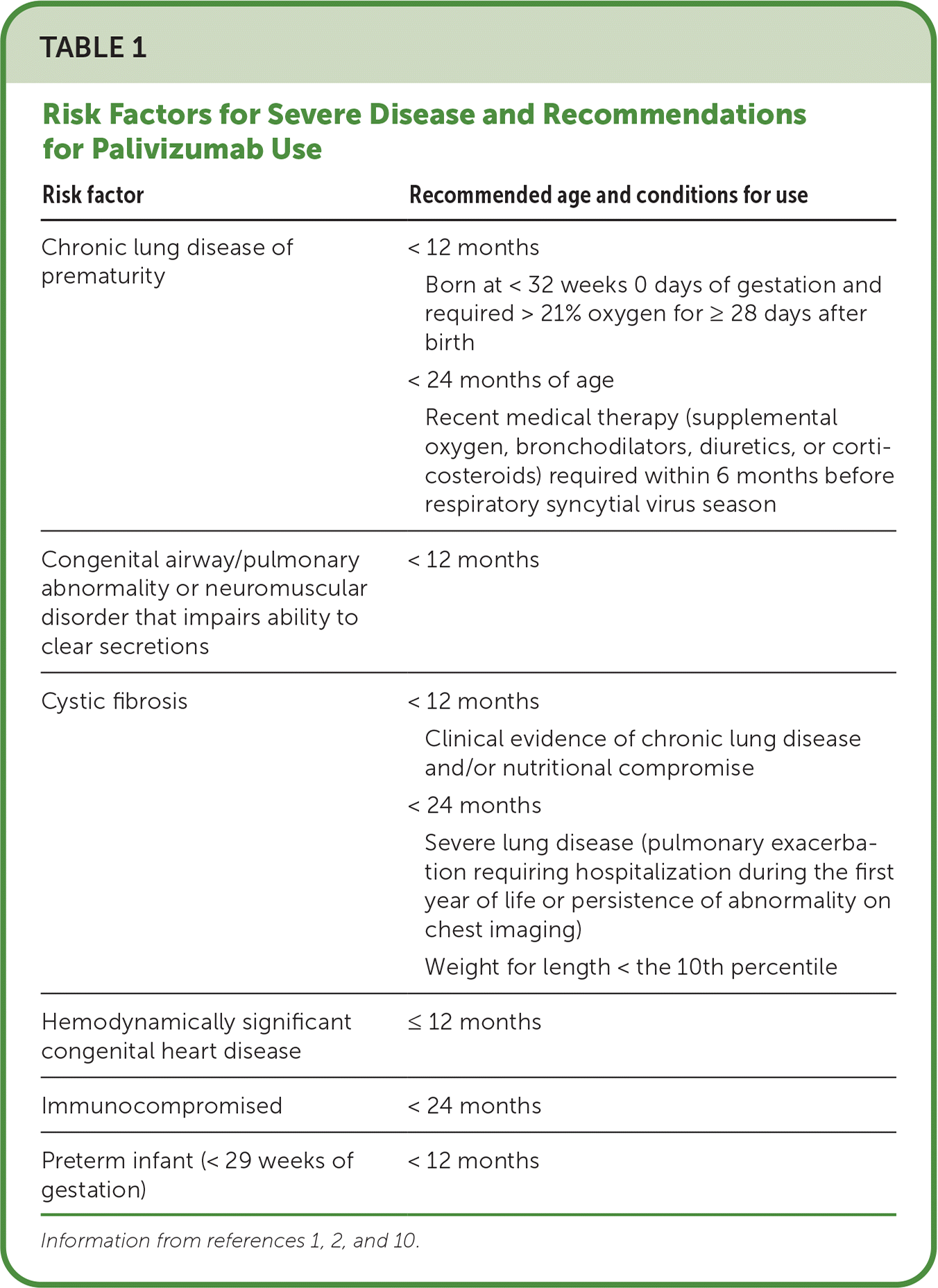
| Risk factor | Recommended age and conditions for use |
|---|---|
| Chronic lung disease of prematurity | < 12 months Born at < 32 weeks 0 days of gestation and required > 21% oxygen for ≥ 28 days after birth < 24 months of age Recent medical therapy (supplemental oxygen, bronchodilators, diuretics, or corticosteroids) required within 6 months before respiratory syncytial virus season |
| Congenital airway/pulmonary abnormality or neuromuscular disorder that impairs ability to clear secretions | < 12 months |
| Cystic fibrosis | < 12 months Clinical evidence of chronic lung disease and/or nutritional compromise < 24 months Severe lung disease (pulmonary exacerbation requiring hospitalization during the first year of life or persistence of abnormality on chest imaging) Weight for length < the 10th percentile |
| Hemodynamically significant congenital heart disease | ≤ 12 months |
| Immunocompromised | < 24 months |
| Preterm infant (< 29 weeks of gestation) | < 12 months |
Diagnosis
SIGNS AND SYMPTOMS
Presentation of RSV bronchiolitis includes two to four days of fever and upper respiratory tract symptoms, including rhinorrhea, congestion, and sneezing. The disease often progresses to include lower respiratory tract symptoms of cough, tachypnea, retractions, difficulty feeding, and accessory muscle use 2,10,15–17 (Table 217).
Infants typically present with upper and lower respiratory tract symptoms, fever, decreased appetite, and lethargy.15
Any combination of grunting, nasal flaring, and inter-costal, subcostal, or supraclavicular retractions indicates increased work of breathing.2,10
Auscultation may reveal turbulent airflow with a prolonged expiratory phase, diffuse wheezing, and coarse breath sounds.2,10,15
Apnea rates vary from 1% to 24%, but apnea is reported with many viral etiologies.2,10
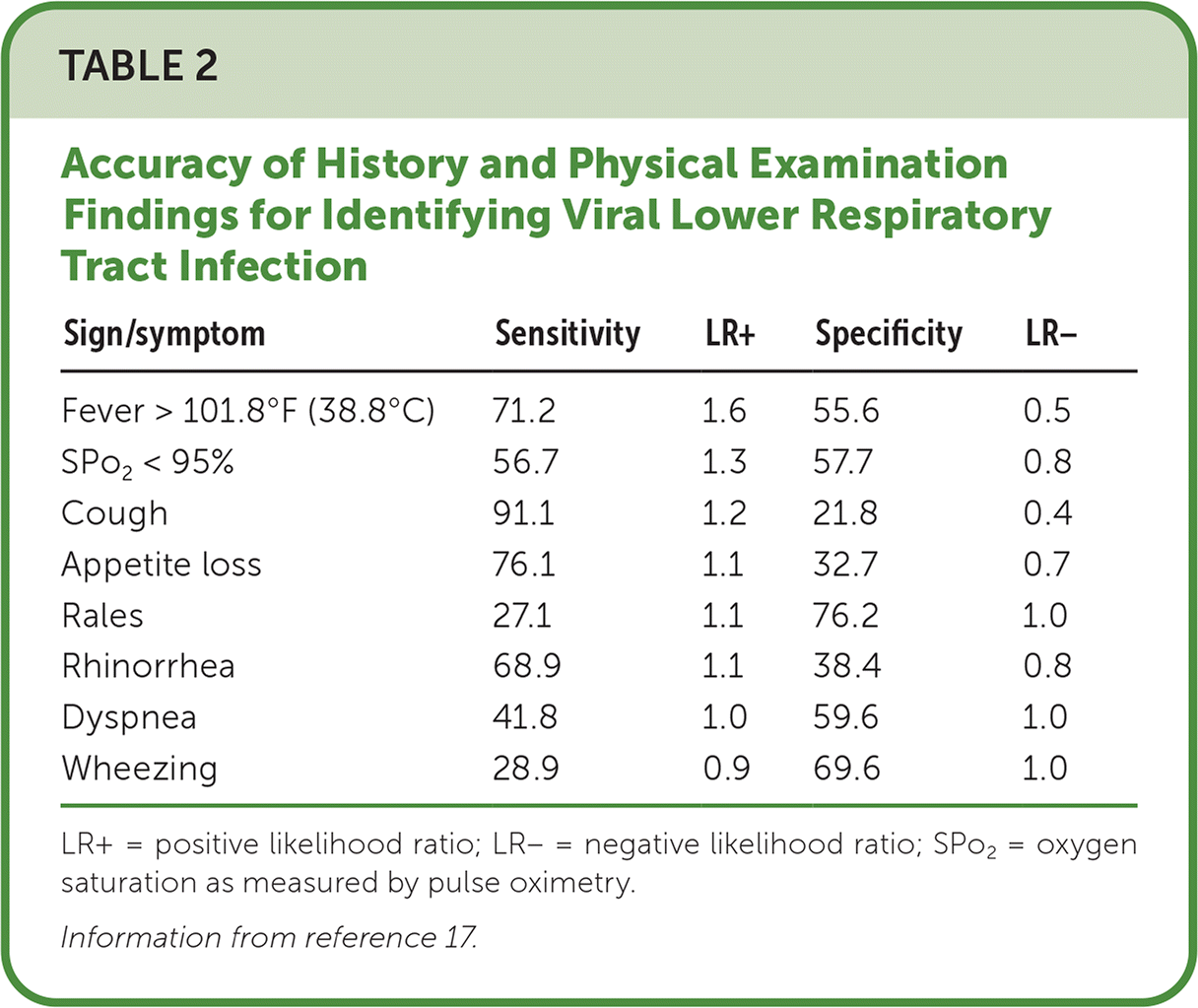
| Sign/symptom | Sensitivity | LR+ | Specificity | LR− |
|---|---|---|---|---|
| Fever > 101.8°F (38.8°C) | 71.2 | 1.6 | 55.6 | 0.5 |
| SPo2 < 95% | 56.7 | 1.3 | 57.7 | 0.8 |
| Cough | 91.1 | 1.2 | 21.8 | 0.4 |
| Appetite loss | 76.1 | 1.1 | 32.7 | 0.7 |
| Rales | 27.1 | 1.1 | 76.2 | 1.0 |
| Rhinorrhea | 68.9 | 1.1 | 38.4 | 0.8 |
| Dyspnea | 41.8 | 1.0 | 59.6 | 1.0 |
| Wheezing | 28.9 | 0.9 | 69.6 | 1.0 |
ASSESSMENT OF SEVERITY
There is no accepted validated tool to assess or predict severe disease from RSV. The 2014 American Academy of Pediatrics guideline, endorsed by the American Academy of Family Physicians, recommends assessing disease severity with history and physical examination.1,18,19
A 2018 retrospective cohort study of 2,722 children with bronchiolitis found that initial oxygen saturation less than 90%, nasal flaring, grunting, retractions, age younger than two months, dehydration, and poor feeding are associated with increased need for respiratory support and hospitalization if more than one factor is present.20
The effectiveness of pulse oximetry in assessing disease severity is unknown. Transient hypoxemia occurs in healthy infants, and reliance on pulse oximetry without a complete clinical evaluation could lead to unnecessary hospitalization.1,21
DIAGNOSTIC TESTING
Radiography should not be used to diagnose uncomplicated RSV bronchiolitis because it does not improve clinical outcomes, decrease antibiotic use, or distinguish between viral and bacterial etiologies. Radiography should be considered for diagnosis of airway complications and if admission to the intensive care unit is needed.1,2,22,23
Viral testing (Table 317,24,25) also should not be used to diagnose uncomplicated RSV bronchiolitis because results do not reduce antibiotic use or diagnostic imaging, or rule out concurrent bacterial infection. Viral testing should be considered for febrile infants younger than three months and children who are immunocompromised, need intensive care unit admission, or are receiving palivizumab when hospitalized (to determine whether continued use is necessary).1,24–28
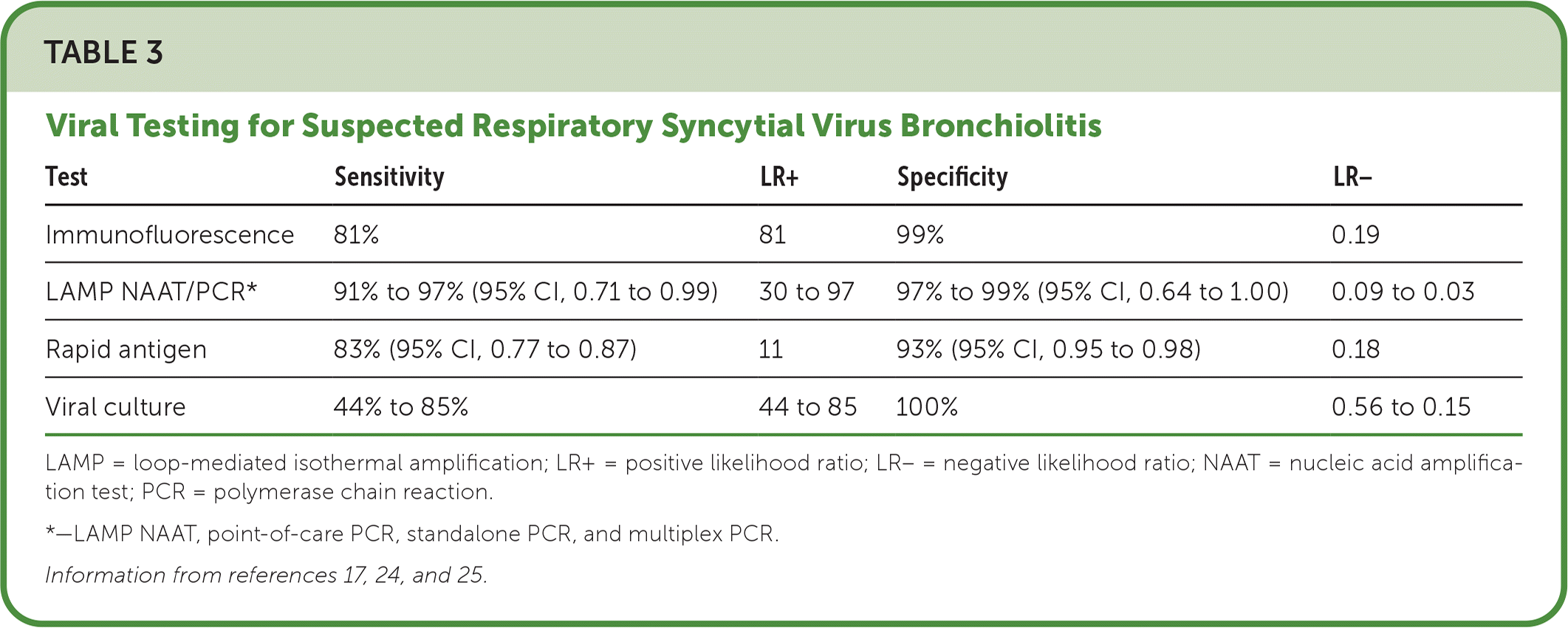
| Test | Sensitivity | LR+ | Specificity | LR− |
|---|---|---|---|---|
| Immunofluorescence | 81% | 81 | 99% | 0.19 |
| LAMP NAAT/PCR* | 91% to 97% (95% CI, 0.71 to 0.99) | 30 to 97 | 97% to 99% (95% CI, 0.64 to 1.00) | 0.09 to 0.03 |
| Rapid antigen | 83% (95% CI, 0.77 to 0.87) | 11 | 93% (95% CI, 0.95 to 0.98) | 0.18 |
| Viral culture | 44% to 85% | 44 to 85 | 100% | 0.56 to 0.15 |
Treatment
Treatment for RSV bronchiolitis is primarily supportive. Hospitalization should be considered for those at risk of requiring increased respiratory support: initial oxygen saturation less than 90% (odds ratio [OR] = 8.9; 95% CI, 5.1 to 15.7), nasal flaring/grunting (OR = 3.8; 95% CI, 2.6 to 5.4), apnea (OR = 3.0; 95% CI, 1.9 to 4.8), retractions (OR = 3.0; 95% CI, 1.6 to 5.7), age two months or younger (OR = 2.1; 95% CI, 1.5 to 3.0), dehydration (OR = 2.1; 95% CI, 1.4 to 3.3), poor feeding (OR = 1.9; 95% CI, 1.3 to 2.7).1,20
Supplemental oxygen via nasal cannula should be administered to maintain oxygen saturation above 90%. Use of a high-flow nasal cannula and continuous positive airway pressure may be considered if more respiratory support is needed to maintain oxygen saturation, although two Cochrane reviews showed that only a few low-quality studies have evaluated these treatments.1,29–31
Use of heliox in early RSV bronchiolitis may improve symptoms but has not been shown to reduce rates of intubation or duration of respiratory support.32
Adequate nutrition and hydration should be ensured, with consideration of intravenous or nasogastric routes if needed.1,33
The American Academy of Pediatrics and National Institute for Health and Care Excellence do not recommend treatment with bronchodilators, epinephrine, nebulized hypertonic saline, corticosteroids, or antibiotics because consistent benefit has not been reported.1,29
A 2014 Cochrane review demonstrated that bronchodilator use does not improve oxygen saturation or reduce hospital admissions, length of hospitalization, or duration of illness. Additionally, use of bronchodilators have adverse effects, including tachycardia, tremor, and oxygen desaturation, and increases cost.34
Nebulized epinephrine in the outpatient setting reduces hospital admissions according to a 2011 Cochrane review (six studies; risk ratio = 0.67; 95% CI, 0.50 to 0.89; NNT = 5), but only if treatment occurs within 24 hours of presentation. There is no evidence to support repeated or prolonged administration. Inpatient administration of nebulized epinephrine is not recommended and has not been shown to reduce length of hospitalization.35,36
A 2023 Cochrane review showed that nebulized hyper-tonic saline may reduce hospital admissions (eight studies; risk ratio = 0.87; 95% CI, 0.78 to 0.97; NNT = 17) and length of hospitalization (21 studies; mean difference = −0.40 days; 95% CI, −0.69 to −0.11) compared with 0.9% saline or standard care. However, quality of evidence was low to very low.37
Systemic or inhaled corticosteroids do not reduce hospital admissions or length of stay.38 One randomized controlled trial of 800 patients showed that combined treatment with dexamethasone and nebulized epinephrine decreases hospital admissions (RR = 0.65; 95% CI, 0.45 to 0.95; NNT = 11); however, its safety and effectiveness have not been established.38,39
Antibiotics should be used only if there is evidence of concurrent bacterial infection.1,40,41 A 2014 Cochrane review demonstrated that treating RSV bronchiolitis with antibiotics, specifically macrolides, does not decrease duration of illness or length of hospitalization.41
Routine use of chest physiotherapy or excessive/deep nasal suction for clearance of secretions is not beneficial, but these interventions may be considered in children with underlying lung or neuromuscular disease.42,43
Prognosis
RSV bronchiolitis is typically self-limited, with U.S. mortality rates of less than 0.1%.44
A large case-control study of 740,418 patients found that a history of RSV bronchiolitis is associated with a threefold increased risk of asthma (4.8% vs. 1.5%; relative risk = 3.1; 95% CI, 2.9 to 3.3). It is not clear whether this association is causal or represents latent reactive airway disease.45
This article updates previous articles on this topic by Smith, et al.46; Dawson-Caswell and Muncie47; and Prasaad Steiner.48
Data Sources: PubMed and the Cochrane database were the primary data sources used to identify literature. Essential Evidence Plus was searched to identify further sources. Primary search terms included respiratory syncytial virus/RSV bronchiolitis free text, and bronchiolitis with one of the following: risk, diagnosis, treatment, prevention. Search dates: June to November 2022, and June 2023.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as the official policy or position of the U.S. Army, U.S. Department of Defense, or the U.S. government.
