
Am Fam Physician. 2023;108(2):189-191
Author disclosure: No relevant financial relationships.
The BioFire Respiratory 2.1 Panel (RP2.1) is a multiplex polymerase chain reaction (PCR) test that identifies nucleic acids from 23 different bacteria and viruses that commonly cause upper and lower respiratory tract infections. It is a point-of-care nasopharyngeal swab that provides quick and accurate results.1 The U.S. Food and Drug Administration granted the BioFire RP2.1 test emergency use authorization in May 2020 and marketing authorization in March 2021, making it the first test including SARS-CoV-2 to be marketed beyond the height of the pandemic.2
Accuracy
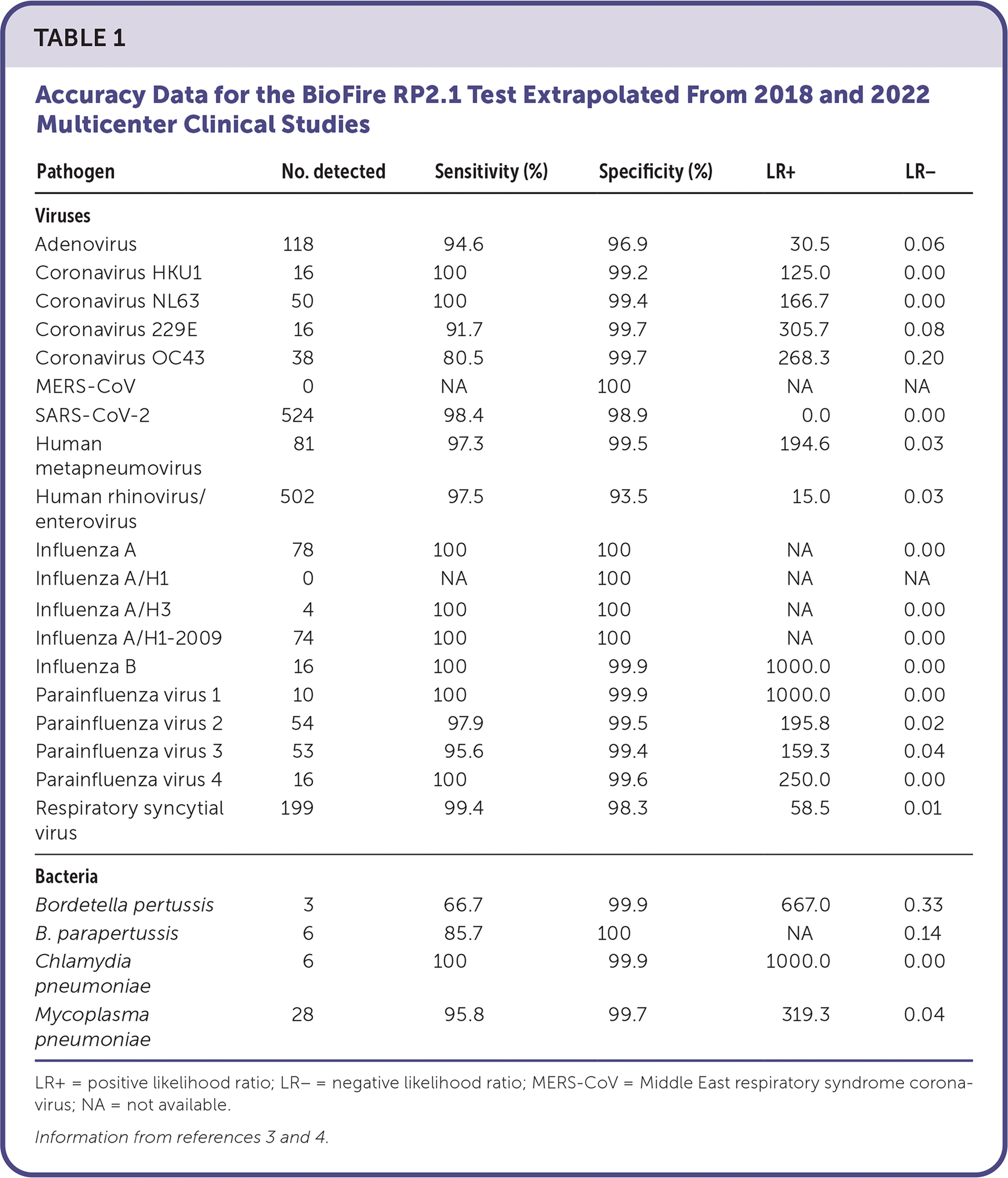
| Pathogen | No. detected | Sensitivity (%) | Specificity (%) | LR+ | LR− |
|---|---|---|---|---|---|
| Viruses | |||||
| Adenovirus | 118 | 94.6 | 96.9 | 30.5 | 0.06 |
| Coronavirus HKU1 | 16 | 100 | 99.2 | 125.0 | 0.00 |
| Coronavirus NL63 | 50 | 100 | 99.4 | 166.7 | 0.00 |
| Coronavirus 229E | 16 | 91.7 | 99.7 | 305.7 | 0.08 |
| Coronavirus OC43 | 38 | 80.5 | 99.7 | 268.3 | 0.20 |
| MERS-CoV | 0 | NA | 100 | NA | NA |
| SARS-CoV-2 | 524 | 98.4 | 98.9 | 0.0 | 0.00 |
| Human metapneumovirus | 81 | 97.3 | 99.5 | 194.6 | 0.03 |
| Human rhinovirus/enterovirus | 502 | 97.5 | 93.5 | 15.0 | 0.03 |
| Influenza A | 78 | 100 | 100 | NA | 0.00 |
| Influenza A/H1 | 0 | NA | 100 | NA | NA |
| Influenza A/H3 | 4 | 100 | 100 | NA | 0.00 |
| Influenza A/H1-2009 | 74 | 100 | 100 | NA | 0.00 |
| Influenza B | 16 | 100 | 99.9 | 1000.0 | 0.00 |
| Parainfluenza virus 1 | 10 | 100 | 99.9 | 1000.0 | 0.00 |
| Parainfluenza virus 2 | 54 | 97.9 | 99.5 | 195.8 | 0.02 |
| Parainfluenza virus 3 | 53 | 95.6 | 99.4 | 159.3 | 0.04 |
| Parainfluenza virus 4 | 16 | 100 | 99.6 | 250.0 | 0.00 |
| Respiratory syncytial virus | 199 | 99.4 | 98.3 | 58.5 | 0.01 |
| Bacteria | |||||
| Bordetella pertussis | 3 | 66.7 | 99.9 | 667.0 | 0.33 |
| B. parapertussis | 6 | 85.7 | 100 | NA | 0.14 |
| Chlamydia pneumoniae | 6 | 100 | 99.9 | 1000.0 | 0.00 |
| Mycoplasma pneumoniae | 28 | 95.8 | 99.7 | 319.3 | 0.04 |
Benefit
Although the BioFire RP2.1 test allows for identification of many common respiratory tract infections in 45 minutes, there is limited clinical benefit. A 2015 observational study of outpatient respiratory pathogen testing used in 295 adults was notable for a decrease in antibiotic use in those who tested positive for influenza.5 However, there was no difference in antibiotic use in those who tested positive for other viruses or negative for all viruses (48.6% and 49.3%, respectively). A 2019 retrospective cohort study of 243 adults discharged from the emergency department and 243 adults who were hospitalized had similar findings.6 In patients discharged from the emergency department, antibiotic use was lower only in those who tested positive for influenza, with an average of 0.5 days of antibiotic therapy compared with 2.7 days for those with negative test results and 1.8 days for those negative for influenza. No statistically significant decrease in antibiotic use occurred in hospitalized patients who received respiratory pathogen testing. In a 2019 randomized controlled study of 800 hospitalized adults, adding respiratory pathogen testing to routine real-time PCR reduced the duration of intravenous antibiotics (seven vs. eight days; P < .001) and shortened the length of hospitalization (eight vs. nine days; P < .001) compared with real-time PCR testing alone.7
In a quasi-randomized trial of 545 hospitalized adults, no statistically significant correlation was identified between respiratory pathogen testing and length of hospitalization or duration of antibiotic use, but there was a decrease in time to first dose of antivirals when using respiratory pathogen testing compared with routine, laboratory-based respiratory PCR and serology testing.8 In a 2016 retrospective, case-control study of 2,430 children, there were decreased antibiotic use (four vs. five median antibiotic days; P < .01) and fewer chest radiographs ordered (59% vs. 78%; P < .01) for those who underwent respiratory pathogen testing, compared with non-PCR methods.9 More than one-half (54.3%) of children whose results were positive for a viral pathogen on respiratory pathogen testing still received antibiotics.
Harms
Although the BioFire RP2.1 test includes 23 different viruses and bacteria, it is not comprehensive. This testing can lead to anchoring bias, and an initial positive result may lead to the absence of a broader differential. Positive results do not rule out coinfection from other organisms not being tested.
Cost
The BioFire RP2.1 test is authorized for use on the BioFire FilmArray 2.0 and BioFire Torch Systems. The reimbursement rate for the consumable RP2.1 test is $417, according to the 2022 Centers for Medicare and Medicaid Services clinical laboratory fee schedule; however, respiratory panels testing for six or more pathogens are not eligible for this reimbursement, per Medicare policy.10,11 This is in contrast to influenza A and B, SARS-CoV-2, and respiratory syncytial virus multiplex testing, which is fully covered by Medicare at a reimbursement rate of $143.12 Additionally, the capital purchase of the BioFire FilmArray and BioFire Torch Systems can cost $37,500 and $35,000, respectively (Bio-Fire Diagnostics sales, personal communication, December 29, 2022).
Bottom Line
The BioFire RP2.1 is a rapid and accurate test that can assist with timely antiviral therapy and infection control measures, but evidence is mixed over its clinical benefit in decreasing the length of antibiotic use in adults.5–9 In agreement with the Infectious Diseases Society of America 2018 recommendations, broad respiratory pathogen panels should be used only when they affect patient management, such as to alter empiric antimicrobial therapy or to change infection control measures.13 Instead of ordering large multiplex tests, physicians should consider using tests of commonly suspected pathogens based on location, season, and patient history.13 However, immuno compromised and critically ill patients with pneumonia and exacerbations of airway disease may benefit from the use of the BioFire RP2.1 test, which is endorsed by the Infectious Diseases Society of America's 2020 diagnostic committee.14
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army, the U.S. Department of Defense, or the U.S. government.
