
Am Fam Physician. 2023;108(3):260-266
Related Letter to the Editor: Timely and Effective Outpatient Benzodiazepine Withdrawal Protocol
Published online August 11, 2023.
Author disclosure: No relevant financial relationships.
In the United States, more than 30 million adults have reported taking a benzodiazepine within the past year. Misuse—use of a drug in a way that a doctor did not direct—accounts for 17.2% of all benzodiazepine use. Family physicians face challenges when balancing the patient's perceived benefits of benzodiazepines with known risks and lack of evidence supporting their use. Benzodiazepines cause significant central nervous system–related adverse effects including sedation, confusion, memory loss, depression, falls, fractures, and motor vehicle crashes. Factors that increase the risk of adverse effects and misuse are other substance use disorders, using concomitant central nervous system medications, and central nervous system or pulmonary diseases. Compared with intermittent use, chronic daily use in older adults is associated with a higher risk of falls, fractures, hospitalizations, and death. Withdrawal symptoms such as anxiety, sleep disturbances, and agitation are common and often prolonged. Adjunctive treatment with antiepileptics, antidepressants, and pregabalin has been shown to lessen withdrawal symptoms. Deprescribing benzodiazepines for patients who use them chronically should be individualized with slow tapering over weeks to months, or longer, to minimize the intensity of withdrawal symptoms. Incorporating behavioral interventions, such as cognitive behavior therapy, improves deprescribing outcomes.
In the United States between 2014 and 2016, nearly one-half (48%) of the estimated 65.9 million office visits per year where benzodiazepines were prescribed were primary care visits, and most were continued prescriptions.1 The rapid anxiolytic and sedative properties of benzodiazepines make them an attractive option for treating acute anxiety and insomnia, but data are lacking to support ongoing therapy for more than one month.2 Therefore, family physicians face challenges when balancing the patient's perceived benefits of benzodiazepines with known risks and lack of evidence supporting their use. Because of their potent agonistic activity on the gamma-aminobutyric acid receptors in the brain and periphery, benzodiazepines cause receptor downregulation within weeks of use.3 Tolerance to anxiolytic and hypnotic effects can develop soon after, with physiologic and psychological dependence. This dependence often leads to continued benzodiazepine use to abate withdrawal.4
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| Avoid chronic benzodiazepine use to prevent serious adverse effects including depression, falls, motor vehicle crashes, and cognitive deficits or dementia.16,19–23 | B | Meta-analysis of lower-quality cohort studies |
| Cognitive behavior therapy with gradual tapering may increase the success of deprescribing benzodiazepines compared with tapering alone.31 | B | Randomized controlled trials of limited-quality patient-oriented evidence |
| Consider adjunct pharmacotherapy using tricyclic antidepressants, paroxetine, carbamazepine, or pregabalin (Lyrica) to facilitate benzodiazepine tapering and reduce withdrawal symptoms.35 | B | Meta-analysis of lower-quality randomized controlled trials |
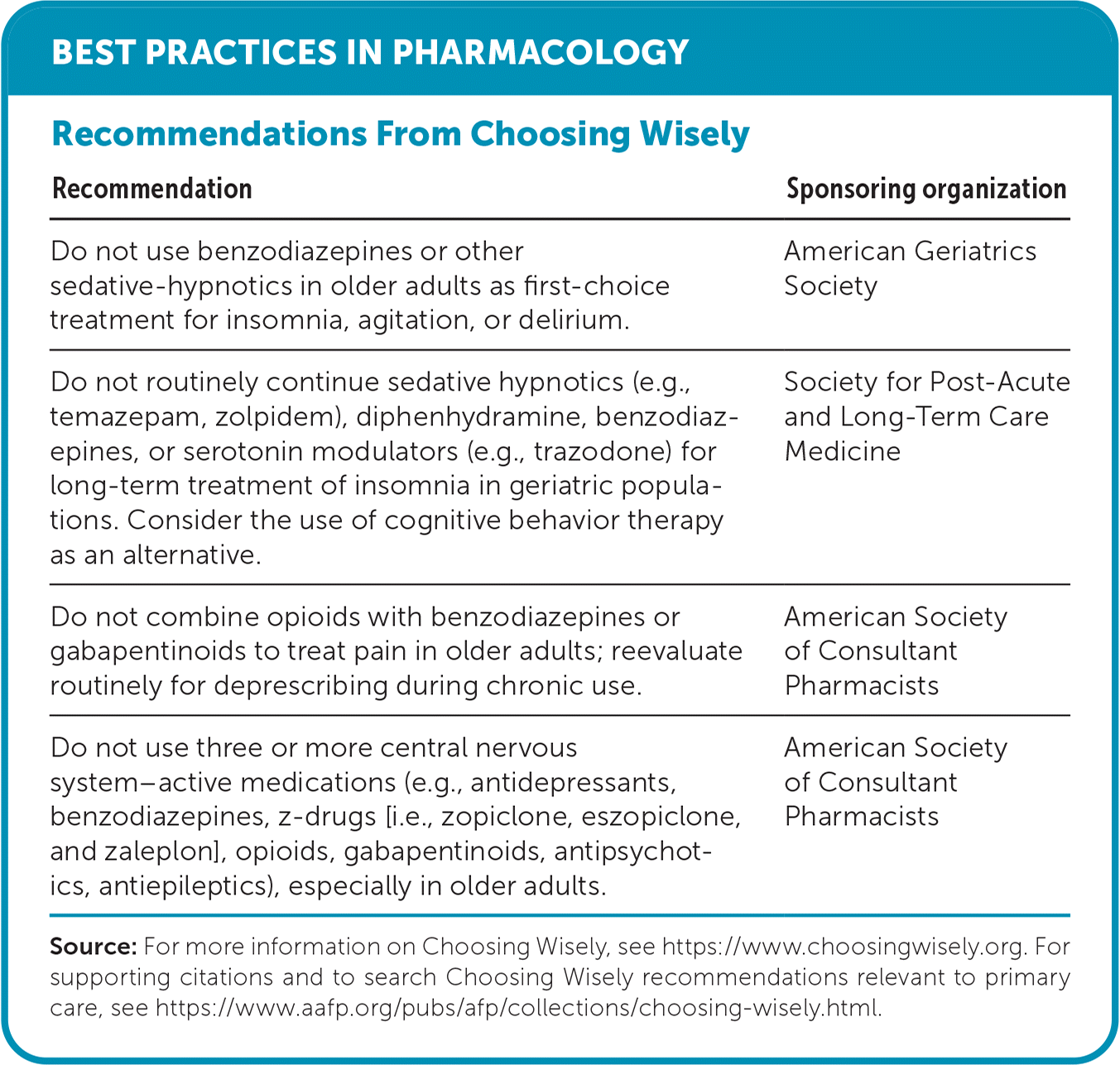
| Recommendation | Sponsoring organization |
|---|---|
| Do not use benzodiazepines or other sedative-hypnotics in older adults as first-choice treatment for insomnia, agitation, or delirium. | American Geriatrics Society |
| Do not routinely continue sedative hypnotics (e.g., temazepam, zolpidem), diphenhydramine, benzodiazepines, or serotonin modulators (e.g., trazodone) for long-term treatment of insomnia in geriatric populations. Consider the use of cognitive behavior therapy as an alternative. | Society for Post-Acute and Long-Term Care Medicine |
| Do not combine opioids with benzodiazepines or gabapentinoids to treat pain in older adults; reevaluate routinely for deprescribing during chronic use. | American Society of Consultant Pharmacists |
| Do not use three or more central nervous system–active medications (e.g., antidepressants, benzodiazepines, z-drugs [i.e., zopiclone, eszopiclone, and zaleplon], opioids, gabapentinoids, antipsychotics, antiepileptics), especially in older adults. | American Society of Consultant Pharmacists |
Identifying and diagnosing benzodiazepine use disorder can be challenging. In 2013, the Diagnostic and Statistical Manual of Mental Disorders, 5th ed., (DSM-5) combined the individual diagnoses of substance abuse and substance dependence into a single diagnosis of substance use disorder. Substance use disorders are defined by pathologic behaviors that cause an individual to continue to use a substance despite experiencing significant problems related to that substance. The DSM-5, text revision categorizes benzodiazepine use disorder as a subtype of the broader category of sedative, hypnotic, or anxiolytic use disorder, but this article will focus only on the benzodiazepine subtype.
The diagnosis of benzodiazepine use disorder is made using 11 criteria that fall into broad categories of impaired control, social impairment, hazardous use, and pharmacologic effects (Table 1).5 The disorder can be further classified by severity depending on the number of criteria met, with only two criteria required for a diagnosis of mild benzodiazepine use disorder.5 Although the behaviors that characterize misuse—use of a drug in a way that a doctor did not direct—are sometimes associated with benzodiazepine use disorder, some (e.g., diversion, nonmedical use, use without a prescription) do not strictly fall within the diagnostic criteria for the disorder. Nevertheless, the potential for misuse of benzodiazepines should be considered by prescribing physicians. The 2015–2016 National Survey on Drug Use and Health showed that 12.6% of the U.S. population (30.6 million people) had used benzodiazepines in the past year, and misuse accounted for 17.2% of use overall. Nearly 7 in 10 people who reported misuse obtained benzodiazepines from a friend or relative. Adults 18 to 25 years of age have the highest rate of misuse compared with older adults.6

Note: This criterion is not considered to be met for individuals taking sedatives, hypnotics, or anxiolytics under medical supervision. Specify current severity: Mild: Presence of 2–3 symptoms Moderate: Presence of 4–5 symptoms Severe: Presence of 6 or more symptoms |
What Are the Risk Factors for Benzodiazepine Misuse and Long-term Use?
An existing or past substance use disorder is the largest risk factor for benzodiazepine misuse. Younger age, chronic sleep disorders, and chronic illness are also associated with benzodiazepine misuse.7,8 Long-term (more than 12 consecutive weeks) benzodiazepine use is more likely in older individuals and those with comorbid conditions.9
EVIDENCE SUMMARY
Substance use disorder is the most significant risk factor for benzodiazepine misuse; 69.5% of those with opioid use disorder, 27.1% with alcohol use disorder, and 77.7% with combined opioid and alcohol use disorder admit to misusing benzodiazepines in their lifetimes.10 Misuse of or dependence on any of these substances, plus using prescription opioids or stimulants, increases the risk even further.7,8,11–14
Many demographic characteristics have been studied to determine the likelihood of benzodiazepine misuse and long-term use (Table 27–14). Lifestyle factors associated with chronic benzodiazepine use, defined as prescriptions for two or more consecutive years without pause, include smoking (odds ratio [OR] = 1.8; 95% CI, 1.6 to 2.1), alcohol use (OR = 1.5; 95% CI, 1.2 to 2.0), and sleep difficulties (OR = 1.4; 95% CI, 1.4 to 1.5), whereas exercise seemed to protect against chronic use (OR = 0.9; 95% CI, 0.88 to 0.96).15
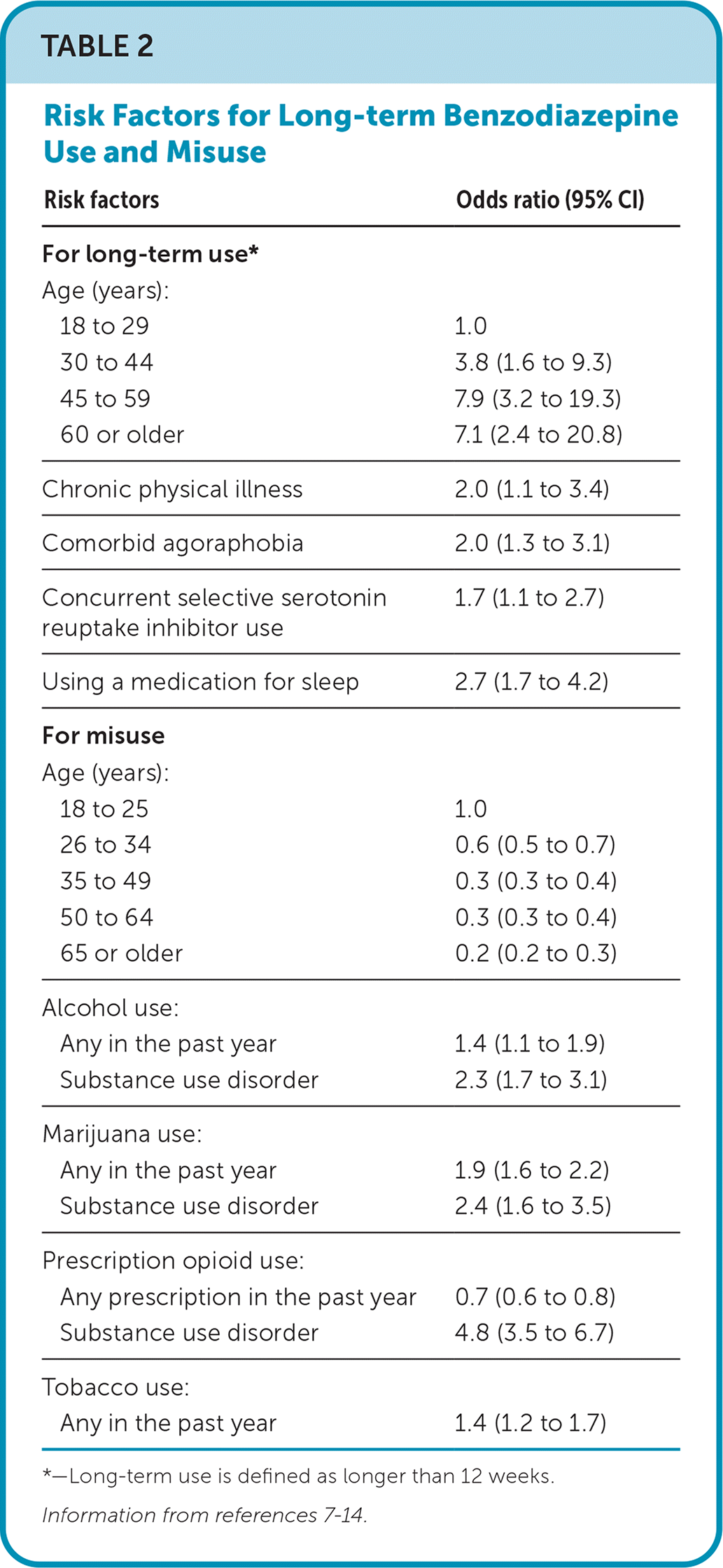
| Risk factors | Odds ratio (95% CI) |
|---|---|
| For long-term use* | |
| Age (years): | |
| 18 to 29 | 1.0 |
| 30 to 44 | 3.8 (1.6 to 9.3) |
| 45 to 59 | 7.9 (3.2 to 19.3) |
| 60 or older | 7.1 (2.4 to 20.8) |
| Chronic physical illness | 2.0 (1.1 to 3.4) |
| Comorbid agoraphobia | 2.0 (1.3 to 3.1) |
| Concurrent selective serotonin reuptake inhibitor use | 1.7 (1.1 to 2.7) |
| Using a medication for sleep | 2.7 (1.7 to 4.2) |
| For misuse | |
| Age (years): | |
| 18 to 25 | 1.0 |
| 26 to 34 | 0.6 (0.5 to 0.7) |
| 35 to 49 | 0.3 (0.3 to 0.4) |
| 50 to 64 | 0.3 (0.3 to 0.4) |
| 65 or older | 0.2 (0.2 to 0.3) |
| Alcohol use: | |
| Any in the past year | 1.4 (1.1 to 1.9) |
| Substance use disorder | 2.3 (1.7 to 3.1) |
| Marijuana use: | |
| Any in the past year | 1.9 (1.6 to 2.2) |
| Substance use disorder | 2.4 (1.6 to 3.5) |
| Prescription opioid use: | |
| Any prescription in the past year | 0.7 (0.6 to 0.8) |
| Substance use disorder | 4.8 (3.5 to 6.7) |
| Tobacco use: | |
| Any in the past year | 1.4 (1.2 to 1.7) |
What Adverse Effects Are Commonly Associated With Benzodiazepine Use and Which Patients Are at Higher Risk?
Harmful short- and long-term adverse effects of benzodiazepines are common and should be the key to counseling patients and justifying limitations in prescribing. The most common adverse effects are sedation, confusion, memory loss, paradoxical anxiety, or worsening depression. No patient is at low risk of adverse effects, but people 65 years or older; those with chronic central nervous system, mental, or pulmonary disease; and people with concomitant use of other central nervous system depressants are at a higher risk of serious adverse effects, such as motor vehicle crashes, hip fractures, or death.4,16–23
EVIDENCE SUMMARY
Central nervous system depression and psychomotor impairments are common adverse effects of benzodiazepines with short- and long-term use. Although these adverse effects may improve slightly with continued use, cognitive impairments, depression,3 and paradoxical anxiety can develop after only one month of continuous use.11 Table 3 lists the most common adverse effects and factors among those at highest risk.2,4,6,7,11,17,20–23
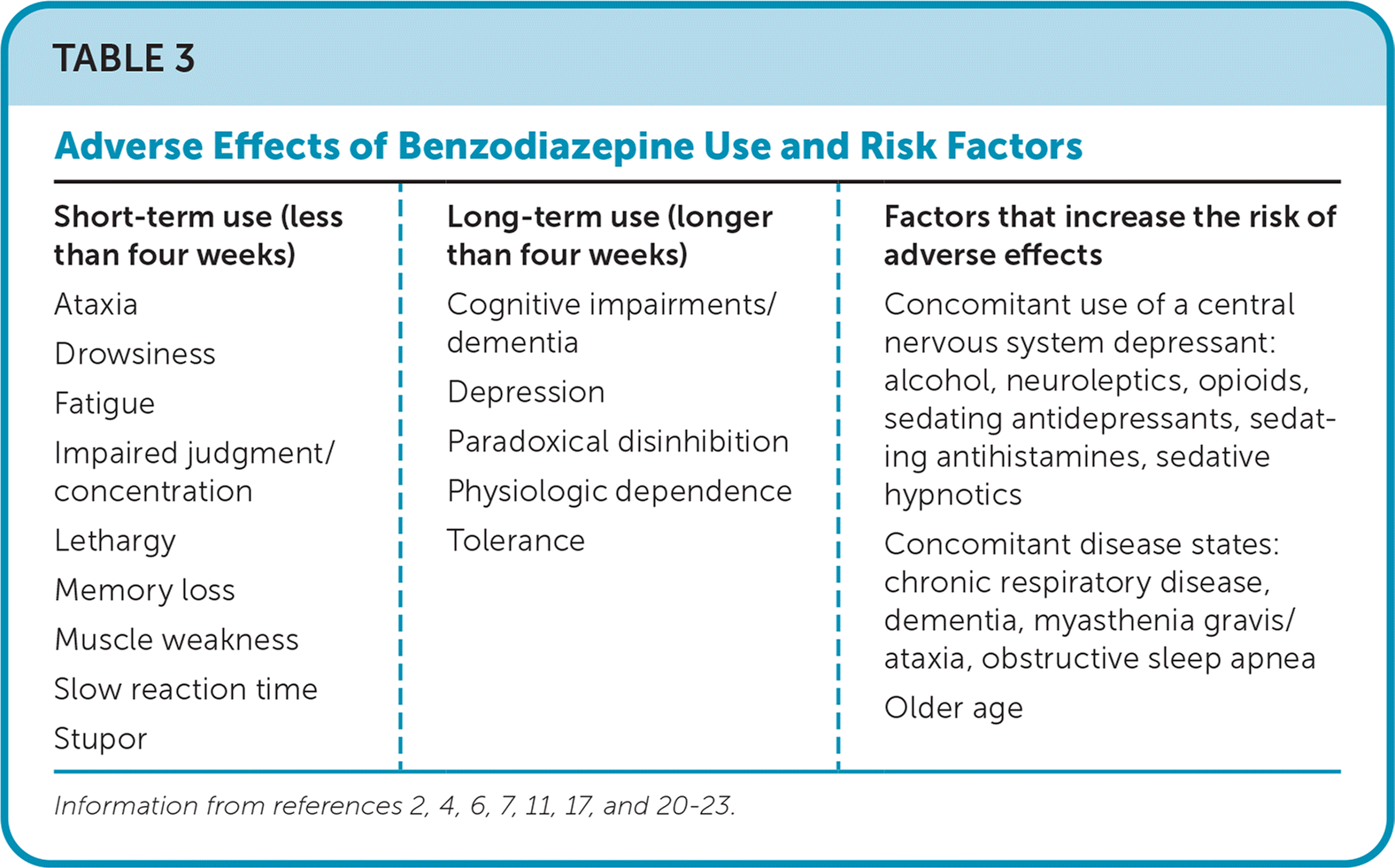
| Short-term use (less than four weeks) Ataxia Drowsiness Fatigue Impaired judgment/concentration Lethargy Memory loss Muscle weakness Slow reaction time Stupor | Long-term use (longer than four weeks) Cognitive impairments/dementia Depression Paradoxical disinhibition Physiologic dependence Tolerance | Factors that increase the risk of adverse effects Concomitant use of a central nervous system depressant: alcohol, neuroleptics, opioids, sedating antidepressants, sedating antihistamines, sedative hypnotics Concomitant disease states: chronic respiratory disease, dementia, myasthenia gravis/ataxia, obstructive sleep apnea Older age |
Chronic benzodiazepine use can worsen symptoms of depression. The proposed mechanisms of action are decreased gamma-aminobutyric acid, monoaminergic function, and serotonin activity.3 One study of 1,200 patients with major depressive disorder found higher rates of anhedonia with concomitant antidepressant and benzodiazepine use at four months compared with antidepressant treatment alone.16
Benzodiazepines a lone rarely lead to overdose and suicides, but when they are combined with alcohol or opioids, profound respiratory depression and death can occur. As many as 70% of patients who died from an opioid overdose also ingested a benzodiazepine.17 Among patients with alcohol use disorder seeking inpatient treatment, 19% reported misuse of a benzodiazepine in the past 30 days.18
Falls, fractures, and motor vehicle crashes are strongly associated with benzodiazepine use. One meta-analysis found that fatal motor vehicle crashes were 2.3 times more likely when the driver was taking a benzodiazepine compared with nonusers.19 Older patients taking benzodiazepines, even for short periods, have a 20% to 40% higher relative risk (RR) for hip fractures compared with nonusers.20
Studies investigating dementia and cancer risk with benzodiazepine use have yielded conflicting results. A recent meta-analysis of benzodiazepine use and dementia suggests a positive association (OR = 1.4; 95% CI, 1.07 to 1.8) but with low certainty.21 A prospective population study in older adults showed an increased risk of dementia and Alzheimer disease with low-dose benzodiazepine exposure (hazard ratio [HR] = 1.25; 95% CI, 1.03 to 1.5), but not with high-dose exposure.22 The dose was determined to be high or low based on cumulative exposure over 10 years, with exposure of more than 120 days defined as high and exposure of one to 30 days as low. A meta-analysis of 19 trials found significant negative cognitive effects (e.g., working memory, processing speed, divided attention) in current long-term users (mean use = 8.1 years). Most cognitive deficits persisted after patients discontinued benzodiazepines for up to 42 months post-withdrawal.23 Observational studies have found a correlation between cancer and benzodiazepine use, but definitive evidence is lacking.20
When and How Should Benzodiazepines Be Tapered?
Deprescribing benzodiazepines using a multifaceted approach should be a goal for all patients. Slow tapering (over weeks to months) is often needed for patients who have been taking benzodiazepines daily for more than one month to minimize withdrawal symptoms and treat underlying disorders. Incorporating behavioral interventions, such as cognitive behavior therapy, improves deprescribing outcomes.
EVIDENCE SUMMARY
Successful deprescribing of benzodiazepines for patients who use them long-term is complex and time consuming. Patient education on the potential benefits of tapering (e.g., improved cognition and psychomotor functioning) can increase the likelihood of successful deprescribing by enhancing patient motivation and self-efficacy. Anticipatory guidance for withdrawal symptoms and allowing patients to actively participate in the tapering process improves outcomes.24,25 Gradual tapers will lessen withdrawal symptoms, but determining the optimal dosing changes involves frequent and open dialogue between physician and patient.
Evidence is lacking to support one tapering regimen over another. Switching from a short-acting benzodiazepine to a long-acting option, such as diazepam, before tapering is a widely accepted protocol based on expert opinion. One small randomized controlled trial of 63 patients, 21 to 75 years of age, with more than one year of benzodiazepine use compared withdrawal symptoms with gradual tapering (25% per week) of a short-acting vs. long-acting benzodiazepine. If withdrawal symptoms were intolerable, tapering rates and period were extended at weekly intervals. Overall, 90% of patients experienced mild to moderate withdrawal symptoms, with no difference in withdrawal symptoms between groups. At the conclusion of the study, 68% of patients in the long-acting group and 58% in the short-acting group were drug free.26
Current practice standards range from converting a short-acting to a long-acting benzodiazepine before tapering (British National Formulary)27 to continuing the same benzodiazepine while tapering (Canadian clinical practice guidelines).28 There are three important aspects of a tapering regimen: (1) dose reduction percentages should be individualized and high maintenance doses can be decreased up to 25% initially, with lower doses requiring 5% to 15% reductions; (2) adjustments require flexibility and often need two to four weeks before the next taper; (3) doses may need to be modified if withdrawal symptoms become problematic, especially at the lower end of the taper.2,11,25,29
Psychotherapy is an important tool when tapering benzodiazepines. Motivational interviewing and other brief interventions can improve outcomes, and cognitive behavior therapy has the best evidence.10,30 Patients 65 years and older tapering long-term benzodiazepines for sleep disorders achieved higher rates of discontinuation at eight weeks, three months, and 12 months with weekly 90-minute cognitive behavior therapy for eight weeks compared with tapering alone (70% vs. 24%; number needed to treat = 2).31 Moderate-quality evidence from 11 studies (575 participants) showed successful discontinuation of benzodiazepines at three months with cognitive behavior therapy plus tapering vs. tapering alone (RR = 1.51; 95% CI, 1.15 to 1.98). This benefit was not sustained at six months in the few studies that followed up for this period.32
How Should Benzodiazepine Withdrawal Symptoms Be Managed?
The clinical presentation of benzodiazepine withdrawal varies. Common symptoms are presented inTable 4.26,28,33,34 Abrupt discontinuation may result in severe withdrawal symptoms, which in rare cases can cause seizures, psychosis, and death.33 Although no medications are approved by the U.S. Food and Drug Administration for the management of benzodiazepine withdrawal syndrome, low-quality evidence exists for the use of antiepileptics, antidepressants, and pregabalin (Lyrica) to increase benzodiazepine discontinuation and reduce withdrawal symptoms.35
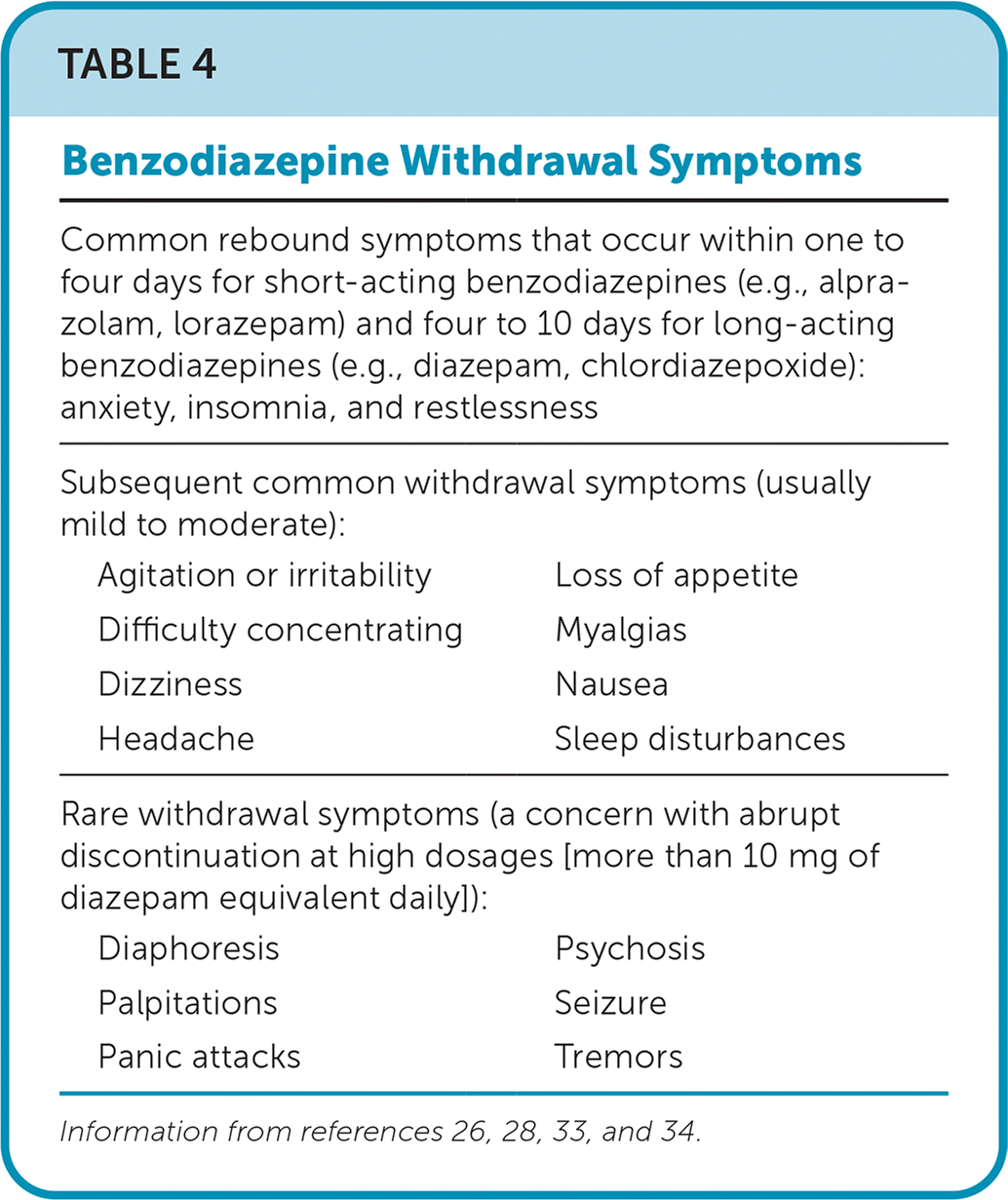
| Common rebound symptoms that occur within one to four days for short-acting benzodiazepines (e.g., alprazolam, lorazepam) and four to 10 days for long-acting benzodiazepines (e.g., diazepam, chlordiazepoxide): anxiety, insomnia, and restlessness | |
| Subsequent common withdrawal symptoms (usually mild to moderate): | |
| Agitation or irritability | Loss of appetite |
| Difficulty concentrating | Myalgias |
| Dizziness | Nausea |
| Headache | Sleep disturbances |
| Rare withdrawal symptoms (a concern with abrupt discontinuation at high dosages [more than 10 mg of diazepam equivalent daily]): | |
| Diaphoresis | Psychosis |
| Palpitations | Seizure |
| Panic attacks | Tremors |
EVIDENCE SUMMARY
A 2018 systematic review of 38 randomized trials including 2,543 adults with chronic benzodiazepine use (defined as more than two months) examined the use of adjunct medications to facilitate benzodiazepine tapering.35 The authors of the review rated the quality of evidence as low to very low because the included trials were small, used poor methodology, and showed a high risk of bias.
Valproate (RR = 2.5; 95% CI, 1.1 to 6.0) and tricyclic anti-depressants (RR = 2.2; 95% CI, 1.3 to 3.9) showed an increase in benzodiazepine discontinuation compared with placebo or no intervention. A reduction in withdrawal symptoms was shown with tricyclic antidepressants (mean difference [MD] = −19.8 points; 95% CI, −19.3 to −20.2 points), paroxetine (MD = −6.7 points; 95% CI, −3.9 to −9.6 points), carbamazepine (MD = −6 points; 95% CI, −2.4 to −9.6 points), and pregabalin (MD = −4.8 points; 95% CI, −4.3 to −5.3 points) using the Physician Withdrawal Checklist (PWC-20) questionnaire (0 to 60 points). No benefit was seen with lithium, buspirone, melatonin, or progesterone.35
How Does a Physician Safely Prescribe Benzodiazepines?
Benzodiazepines are generally considered safe for short-term use (less than one month). There is no compelling evidence to support the safety of long-term use. Physicians should first assess risk factors for misuse and adverse effect potential. Patients should be educated about the risk of dependence from long-term use. Patients with alcohol use disorder or who take opioids chronically should not take benzodiazepines concomitantly. For patients who already use benzodiazepines chronically, physicians should initiate shared decision-making about risks, deprescribing, and alternative options, especially for patients older than 65 years. For patients who continue using benzodiazepines, frequent visits should occur for continued risk and benefit assessment. Relative to intermittent use, chronic use in older adults increases the risk of falls, hospitalization, emergency department visits, hip fractures, long-term care admissions, and death.
EVIDENCE SUMMARY
Risk factors for misuse, adverse effects, withdrawal, and tapering have been described in the preceding sections. A retrospective popu lation-based study found that chronic use (120 days or more of exposure within 180 days) compared with intermittent use (less than 120 days of exposure) in community-dwelling adults older than 65 years was associated with an increased risk of falls leading to hospitalization or emergency department visits (HR = 1.1; 95% CI, 1.1 to 1.2), hip fracture (HR = 1.3; 95% CI, 1.1 to 1.5), long-term care admission (HR = 1.4; 95% CI, 1.2 to 1.5), hospitalization or emergency department visits for any reason (HR = 1.1; 95% CI, 1.1 to 1.14), and mortality (HR = 1.3; 95% CI, 1.2 to 1.4). The differences in each of these outcomes remained after adjusting for mean daily benzodiazepine dosage.36
This article updates a previous article on this topic by Longo and Johnson.4
Data Sources: A PubMed search was completed in Clinical Queries using the key terms benzodiazepine use disorder, withdrawal syndrome, and deprescribing benzodiazepines. The search included meta-analyses, randomized controlled trials, cohort and retrospective trials, and reviews. A search was also performed using Essential Evidence Plus, Dynamed, and Cochrane database. Whenever possible, if studies used race and/or gender as patient categories but did not define how these categories were assigned, they were not included in our final review. Search dates: October and November 2022, and January, February, and June 2023.
