
Am Fam Physician. 2023;108(3):305-306
Author disclosure: No relevant financial relationships.
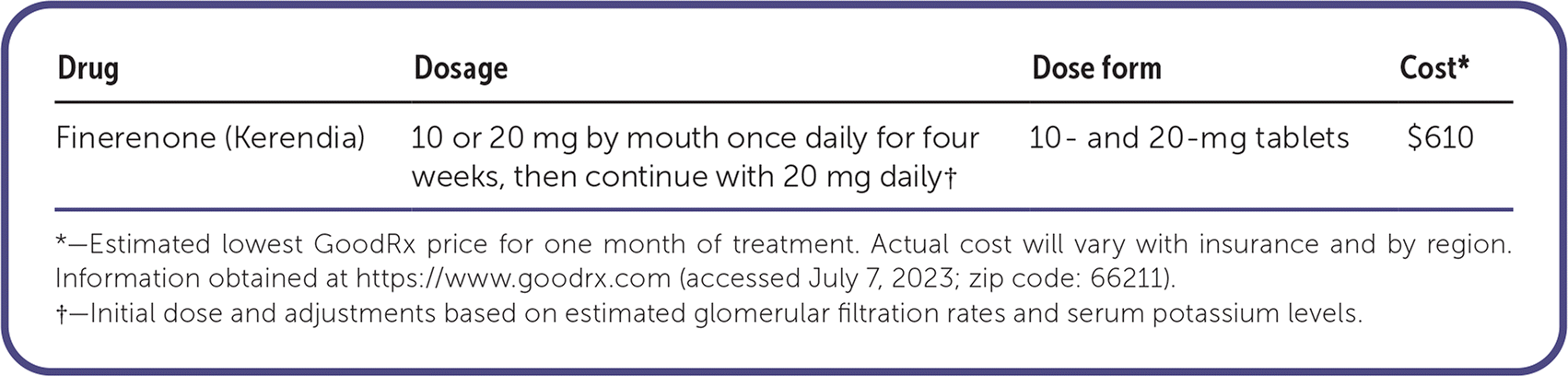
Finerenone (Kerendia) is a nonsteroidal, mineralocorticoid receptor antagonist. It is labeled for the treatment of chronic kidney disease in adults with type 2 diabetes mellitus to reduce the risk of sustained renal function decline, end-stage renal disease, cardiovascular death, nonfatal myocardial infarction, and hospitalization for heart failure.
Safety
Adverse effects of finerenone include hyperkalemia (14%), hypotension (4.6%), and hyponatremia (1.3%).1 Finerenone has not been shown to cause adverse effects associated with spironolactone and eplerenone, such as gynecomastia or breast tenderness. If clinically appropriate, clinicians should avoid using finerenone with other drugs that also increase potassium levels (e.g., nonsteroidal anti-inflammatory drugs, potassium supplements). It should not be used with strong cytochrome P450 3A4 (CYP3A4) inhibitors (e.g., itraconazole, amiodarone, diltiazem, ritonavir) or grapefruit because these increase blood levels and may increase adverse effects. Finerenone should also be avoided with strong or moderate CYP3A4 inducers such as rifampin, phenytoin, carbamazepine, and efavirenz, which decrease the effectiveness of finerenone. There are no available data on the effects of finerenone on pregnancy in humans; however, animal studies have demonstrated embryonic toxicities and suggest that finerenone is present in breast milk; therefore, breastfeeding should be avoided to prevent exposure.1
Tolerability
The most common adverse effect of finerenone is hyperkalemia, which occurs in about 14% of patients and increases as renal function declines. Hyperkalemia leading to hospitalization occurs in less than 1% of patients; however, discontinuation of therapy is necessary in less than 2% of patients if the dosing is adjusted for renal and serum potassium levels. The risk of hyperkalemia is related to baseline renal function and potassium levels. Finerenone should not be initiated when the estimated glomerular filtration rate (GFR) is less than 25 mL per minute or serum potassium is greater than 5.0 mEq per L (5.0 mmol per L).1
Effectiveness
Finerenone was evaluated in two randomized, placebo-controlled trials conducted over three years. These studies included adults with diabetes-related chronic kidney disease who were already taking maximally tolerated dosages of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB). The target daily dosage of finerenone in both studies was 20 mg.1
In 5,674 participants with type 2 diabetes and renal dysfunction (mean baseline estimated GFR of 44 mL per minute and a median urinary albumin-creatinine ratio of 852 mg per g), finerenone reduced the incidence of a composite of kidney failure, renal death, or decline in estimated GFR of at least 40% from baseline by 18% (number needed to treat = 29; 95% CI, 16 to 166) over three years.2 These findings were based primarily on a statistically significant 19% reduction in a sustained estimated GFR decline of at least 40%. Other indicators of kidney function were not affected.
In a second study of 7,437 participants with type 2 diabetes and less severe chronic kidney disease (mean baseline estimated GFR of 68 mL per minute and a median urinary albumin-creatinine ratio of 308 mg per g), additional treatment with finerenone resulted in a 13% reduction (number needed to treat = 47 over 3.5 years; 95% CI, 26 to 226) in the incidence of a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. This composite outcome was driven by a 29% reduction in hospitalization for heart failure. Treatment did not affect cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or the composite of kidney failure, renal death, or decline in estimated GFR of at least 40% from baseline.3
There are no direct comparisons between finerenone and sodium-glucose cotransporter-2 (SGLT-2) inhibitors with similar labeled uses. The effect of finerenone has not been compared with that of spironolactone or eplerenone on kidney disease outcomes. The combination of SGLT-2 inhibitor and finerenone has not been evaluated.
Price
A 30-day supply of finerenone costs about $610. This is comparable to canagliflozin (Invokana), which costs about $620 for a 30-day supply.
Simplicity
Finerenone tablets should be taken orally once daily, with or without food. The starting dosage should be reduced to 10 mg daily in patients with an estimated GFR between 25 and 60 mL per minute. Serum potassium and subsequent potassium level–guided dosing adjustments should be checked at four weeks after initiation (or dose changes) and regularly thereafter. Potassium monitoring is recommended at earlier intervals with concomitant use of weak or moderate CYP3A4 inhibitors or drugs that also affect serum potassium levels. Therapy should be discontinued if mild hyperkalemia (i.e., potassium levels greater than 5.5 mEq per L [5.5 mmol per L]) develops; therapy may be restarted at 10 mg daily once potassium levels are 5.0 mEq per L or less. Dosing titrations to 20 mg daily should be delayed if the serum potassium level is greater than 4.8 mEq per L (4.8 mmol per L) or the estimated GFR decreases by more than 30% from baseline.
Bottom Line
Finerenone slows the decline in renal function in some patients when added to existing therapy. However, it does not affect patient-oriented outcomes in all patients with diabetes-related chronic kidney disease. Finerenone may be added to maximally tolerated doses of ACE inhibitors or ARBs in patients with at least stage 3 chronic kidney disease. Potassium levels should be monitored regularly. The value of finerenone relative to SGLT-2 inhibitors or as an addition to this therapy is not yet known.
