Am Fam Physician. 2024;109(2):143-153
Author disclosure: No relevant financial relationships.
Poisoning is the leading cause of injury-related morbidity and mortality in the United States. The highest rates of exposure to poisons occur in children five years and younger, but opioid overdoses in young adults account for most deaths from poisonings in recent years. Intentional or accidental medication poisoning should be considered when evaluating patients with mental status changes, vital sign abnormalities, seizures, and gastrointestinal or cardiovascular problems. For all poisoned patients, a comprehensive history and physical examination are needed. Knowledge of toxidromes may help identify the cause in unknown ingestions; however, their usefulness may be limited when multiple toxins are ingested. Electrocardiography is indicated in patients reporting chest pain and dyspnea and in overdoses of beta blockers, tricyclic antidepressants, and antidysrhythmics. Measurement of electrolyte, serum creatinine, and serum bicarbonate levels and calculation of the anion gap may be helpful based on the clinical presentation. Treatment of a patient with acute poisoning is based on resuscitation and stabilization with a focus on airway, breathing, and circulation. When poisoning is suspected, the Poison Control provides health care workers and the public with access to a specialist 24 hours a day.
Epidemiology
Poisoning is the primary cause of injury-related deaths in the United States, accounting for 102,001 deaths in 2021 and exceeding motor vehicle collision deaths yearly since 2013.3 The number of deaths from poisonings increased 36% between 2019 and 2021, likely because of the public health crises of the opioid epidemic and worsening of mental health associated with the COVID-19 pandemic.4 Mortality attributable to opioid overdose deaths increased by 41% from 2019 to 2020, with an additional 18% increase in 2021. Adults 35 to 44 years of age account for the most deaths from opioid overdoses of any age group.3
The highest rates of exposure to poisons are associated with exploratory behavior in children five years and younger, accounting for 41.7% of all exposures but only 1.6% of exposure-related fatalities. In contrast, according to the 2021 annual report of the National Poison Data System, 92.5% of poisoning fatalities occurred in adults 20 years and older.2 Table 1 lists the most common substances implicated in children and adult poisoning exposures.2
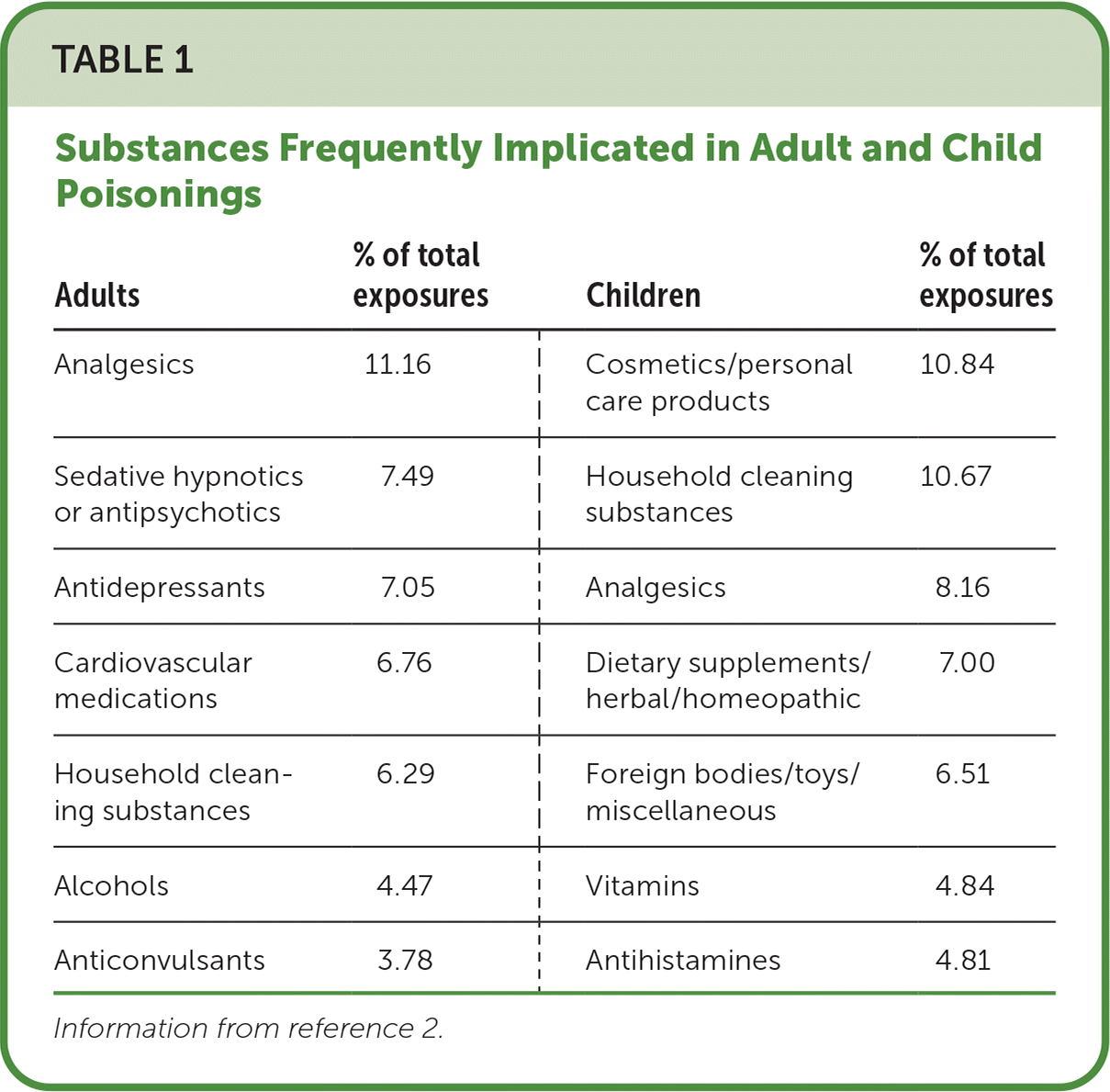
| Adults | % of total exposures | Children | % of total exposures |
|---|---|---|---|
| Analgesics | 11.16 | Cosmetics/personal care products | 10.84 |
| Sedative hypnotics or antipsychotics | 7.49 | Household cleaning substances | 10.67 |
| Antidepressants | 7.05 | Analgesics | 8.16 |
| Cardiovascular medications | 6.76 | Dietary supplements/herbal/homeopathic | 7.00 |
| Household cleaning substances | 6.29 | Foreign bodies/toys/miscellaneous | 6.51 |
| Alcohols | 4.47 | Vitamins | 4.84 |
| Anticonvulsants | 3.78 | Antihistamines | 4.81 |
Initial Approach
Family physicians may be involved in the triage, stabilization, treatment, and disposition of suspected or confirmed poisoning cases; therefore, it is worth considering intentional or accidental medication poisoning when evaluating patients with mental status changes, vital sign abnormalities, seizures, and gastrointestinal or cardiovascular problems. The history and physical examination are important in recognizing the poisoned patient. Knowledge of toxidromes can facilitate recognition of the cause of unknown ingestions, although their usefulness may be limited when multiple toxins are ingested (Table 25–9). When poisoning is suspected, Poison Control (800-222-1222) provides health care workers and the public with access to a specialist 24 hours a day. Poison control centers play an important public health role by reducing unnecessary medical utilization and serving as an epidemiologic surveillance tool.
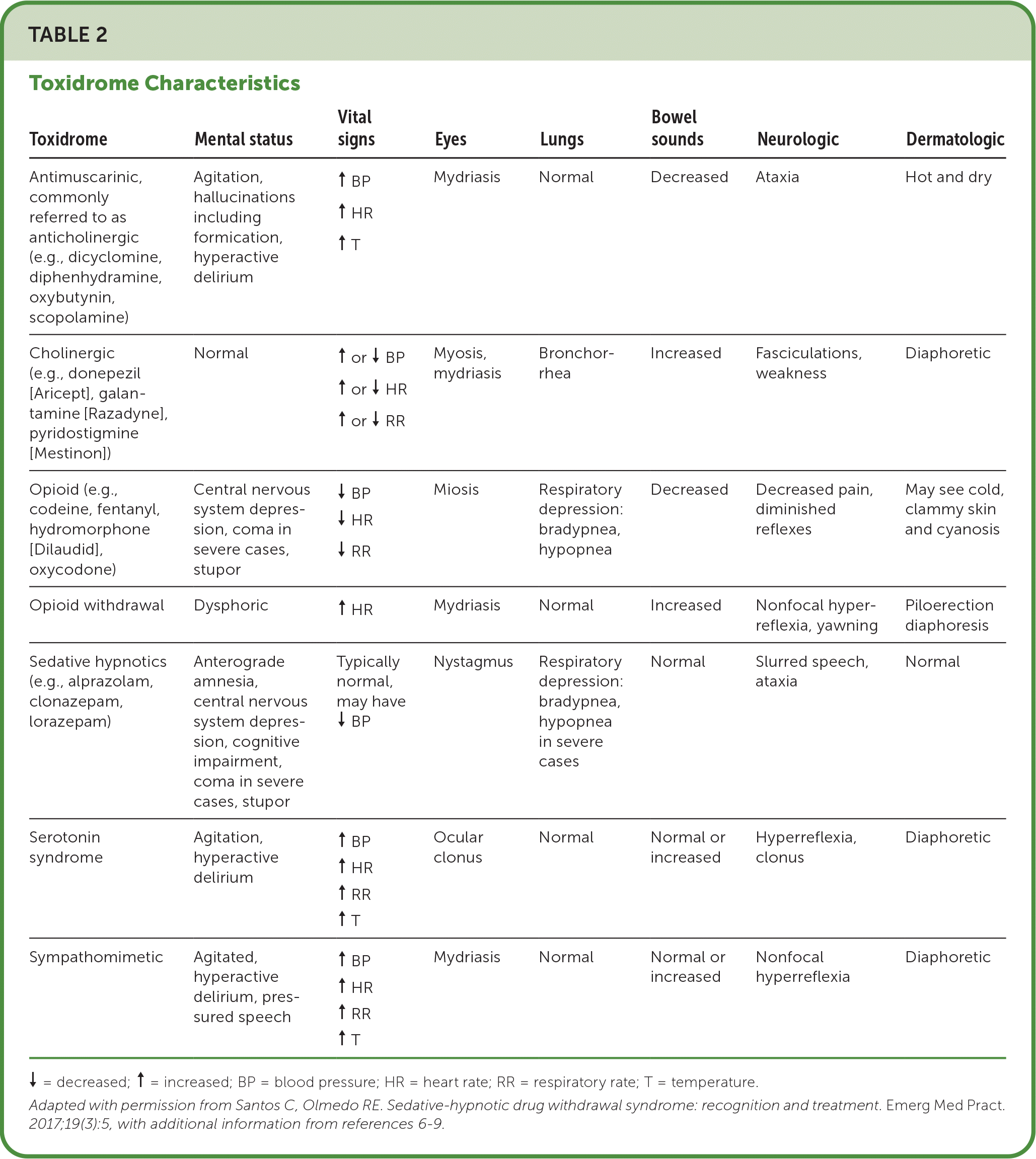
| Toxidrome | Mental status | Vital signs | Eyes | Lungs | Bowel sounds | Neurologic | Dermatologic |
|---|---|---|---|---|---|---|---|
| Antimuscarinic, commonly referred to as anticholinergic (e.g., dicyclomine, diphenhydramine, oxybutynin, scopolamine) | Agitation, hallucinations including formication, hyperactive delirium | ↑ BP ↑ HR ↑ T | Mydriasis | Normal | Decreased | Ataxia | Hot and dry |
| Cholinergic (e.g., donepezil [Aricept], galantamine [Razadyne], pyridostigmine [Mestinon]) | Normal | ↑ or ↓ BP ↑ or ↓ HR ↑ or ↓ RR | Myosis, mydriasis | Bronchorrhea | Increased | Fasciculations, weakness | Diaphoretic |
| Opioid (e.g., codeine, fentanyl, hydromorphone [Dilaudid], oxycodone) | Central nervous system depression, coma in severe cases, stupor | ↓ BP ↓ HR ↓ RR | Miosis | Respiratory depression: bradypnea, hypopnea | Decreased | Decreased pain, diminished reflexes | May see cold, clammy skin and cyanosis |
| Opioid withdrawal | Dysphoric | ↑ HR | Mydriasis | Normal | Increased | Nonfocal hyperreflexia, yawning | Piloerection diaphoresis |
| Sedative hypnotics (e.g., alprazolam, clonazepam, lorazepam) | Anterograde amnesia, central nervous system depression, cognitive impairment, coma in severe cases, stupor | Typically normal, may have ↓ BP | Nystagmus | Respiratory depression: bradypnea, hypopnea in severe cases | Normal | Slurred speech, ataxia | Normal |
| Serotonin syndrome | Agitation, hyperactive delirium | ↑ BP ↑ HR ↑ RR ↑ T | Ocular clonus | Normal | Normal or increased | Hyperreflexia, clonus | Diaphoretic |
| Sympathomimetic | Agitated, hyperactive delirium, pressured speech | ↑ BP ↑ HR ↑ RR ↑ T | Mydriasis | Normal | Normal or increased | Nonfocal hyperreflexia | Diaphoretic |
STABILIZATION
Patients who are symptomatic, are hemodynamically unstable, or have altered mental status with suspected or confirmed exposure should be transported to the emergency department by ambulance. The assessment and initial treatment for a patient with poisoning start with support of the airway, breathing, and circulation. An obstructed airway due to decreased mental status should be addressed with the head tilt–chin lift or modified jaw thrust maneuver. In patients with a loss of airway reflexes or the inability to protect the airway, rapid sequence intubation with rapid onset, short-acting paralytics such as succinylcholine are preferred because long-acting agents could mask toxin-induced seizures. A nondepolarizing paralytic such as rocuronium (Zemuron) should be used for patients with hyperkalemia, digoxin toxicity, or rhabdomyolysis.10
Patients needing transport to another facility may also require intubation if further mental status deterioration is expected. Blood pressure should be measured to assess cardiovascular stability. Hyperthermia and hypothermia associated with poisoning should be managed appropriately with supportive measures. In patients presenting with altered mental status, a point-of-care glucose test should be performed because hypoglycemia is common and easily treated and is a consequence of certain poisonings. If a narcotic overdose is suspected, judicious administration of naloxone should be considered. Benzodiazepines are first-line therapy for nearly all toxin-associated seizures.11
After stabilization, a history should be obtained from the patient or other reliable reporters. Important questions include what substances were ingested, the time of ingestion, and possible amounts taken. Obtaining a history can be challenging in patients who are intoxicated, suicidal, or have an altered mental status. For those patients, family, friends, and emergency medical personnel may be able to provide information such as pictures of medications available at home, current prescriptions, or pill bottles.
PHYSICAL EXAMINATION
A comprehensive physical examination can provide important diagnostic clues in poisonings. After stabilization, a physical examination should be performed with repeated vital signs and possible cardiac monitoring. Patients with poisoning frequently present with neurologic findings such as altered mental status or seizures. The Glasgow Coma Scale can help document and communicate a patient's degree of impairment. Pupillary findings, extraocular movements, tremors, muscle tone, and speech provide important information in poisonings. The gastrointestinal examination should assess for the presence of bowel sounds. The skin examination should note the presence of transdermal patches, track marks, skin color, temperature, and the presence of diaphoresis (typical of sympathomimetic, cholinergic, and serotonin toxicity) or anhidrosis (found in anticholinergic poisoning).11
EVALUATION
Diagnostic testing should be focused on determining the suspected toxins and the potential severity of poisoning. A workup performed in consultation with a regional poison center or toxicologist prevents unnecessary, expensive, and potentially misleading screening. Electrocardiography (ECG) is indicated in patients reporting chest pain and dyspnea and in overdoses that affect beta-adrenergic receptors, sodium, potassium, or calcium channels, such as with beta blockers, tricyclic antidepressants, and antidysrhythmics. ECG is also indicated when there may be unknown coingestions.12 ECG may detect interval abnormalities such as prolonged QRS (classically associated with tricyclic antidepressant overdoses), prolonged QTc (Table 313), and atrioventricular nodal blockade (caused by beta blockers, calcium channel blockers, or digoxin).11 Quantitative serum acetaminophen and salicylate levels should be obtained when ingestion of these substances is suspected. Levels should also be measured in unknown ingestions, when the patient is unconscious, in intentional overdoses because these agents are ubiquitous in many over-the-counter medications, when serum levels correlate with acute toxicity, and if results could help guide treatment.14
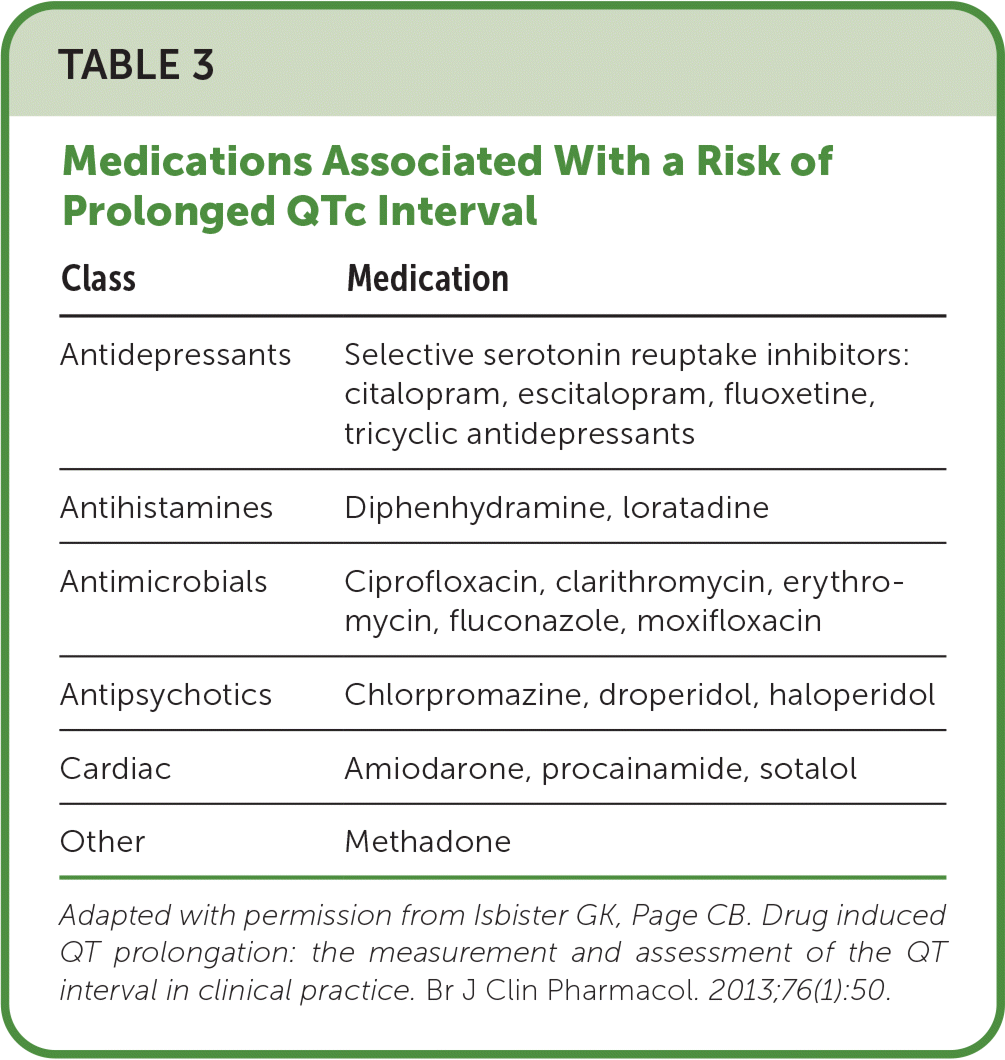
| Class | Medication |
|---|---|
| Antidepressants | Selective serotonin reuptake inhibitors: citalopram, escitalopram, fluoxetine, tricyclic antidepressants |
| Antihistamines | Diphenhydramine, loratadine |
| Antimicrobials | Ciprofloxacin, clarithromycin, erythromycin, fluconazole, moxifloxacin |
| Antipsychotics | Chlorpromazine, droperidol, haloperidol |
| Cardiac | Amiodarone, procainamide, sotalol |
| Other | Methadone |
The measurement of electrolyte, serum creatinine, and serum bicarbonate levels and calculation of the anion gap may be helpful based on the clinical presentation (Table 415). Obtaining blood gas levels helps identify and characterize acidosis or alkalosis. A urine or serum qualitative pregnancy test is recommended in women of childbearing age who present with poisoning or drug overdose. Urine toxicology screening is of limited value in the acute treatment of poisoned patients due to its limited sensitivity, specificity, and unreliable clinical correlation with the presenting clinical scenario.11,14 For example, a negative screen is based on a predetermined cutoff value, does not exclude the presence of a drug, and does not mean the drug was not present in a toxic concentration outside the detection time of the assay. Novel psychoactive substances and research chemicals used by patients typically do not yield positive test results in common toxicologic screens. A positive screen may be due to a false positive or an incidentally noted substance not responsible for the poisoning.11
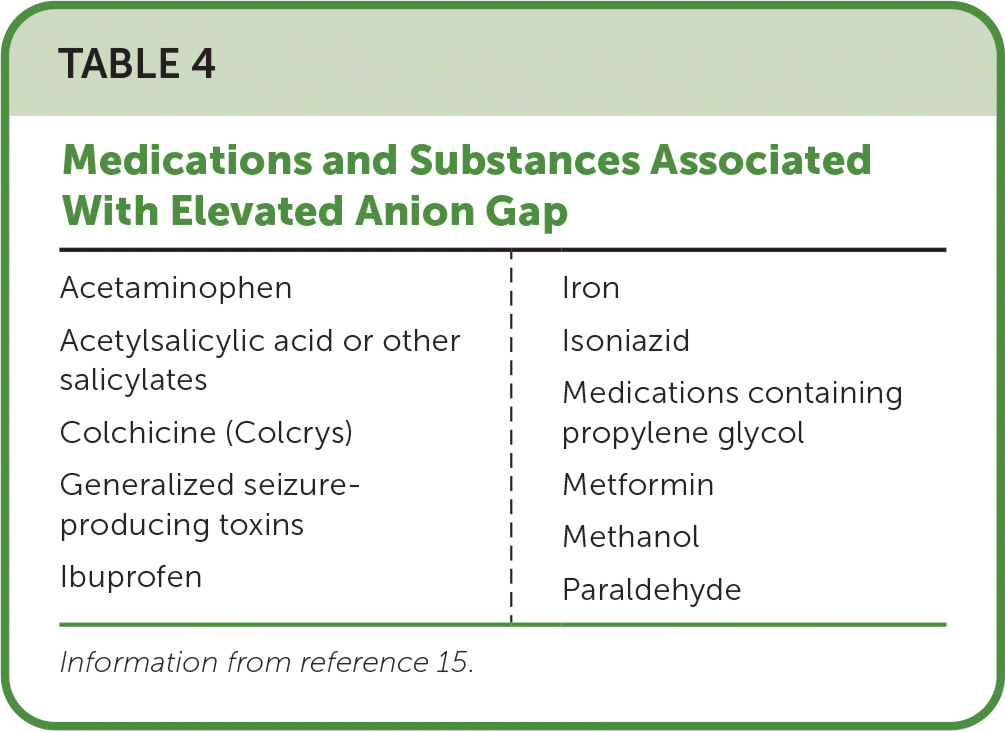
| Acetaminophen Acetylsalicylic acid or other salicylates Colchicine (Colcrys) Generalized seizure-producing toxins Ibuprofen | Iron Isoniazid Medications containing propylene glycol Metformin Methanol Paraldehyde |
Imaging of the chest in patients who are hypoxic, tachypneic, or obtunded may help identify toxin-induced aspiration pneumonitis, acute respiratory distress syndrome, or pulmonary edema. Plain radiography of the abdomen may be useful in large ingestions (e.g., body packing) or radiopaque pills (e.g., iron; neuroleptics; salicylates; iodinated compounds; enteric-coated, sustained-release agents), but it has low sensitivity compared with computed tomography.11,16
Management
Resuscitation and stabilization are the focus of treatment in patients with acute poisoning. Additional interventions require weighing the relative risks of the poisoning against the potential risks and benefits of treatments. For example, the management of pregnant patients with poisoning typically mirrors that of nonpregnant patients. One exception is the use of naloxone for life-threatening opioid overdose near delivery. Opioids and naloxone cross the placenta, and administration of naloxone can precipitate labor, hypertensive crisis, and neonatal abstinence syndrome. Naloxone use near delivery is reserved for patients in which maternal life is threatened; it is administered at the lowest dose necessary to obtain a clinical response.17 Although groups like the American Academy of Clinical Toxicology and American College of Medical Toxicology have guidelines for management, the data supporting many poisoning interventions are based on case reports or retrospective case series due to the inherent challenges of performing randomized controlled trials in humans.18,19 Table 5 lists treatment options for acute medication poisoning.5,20–33
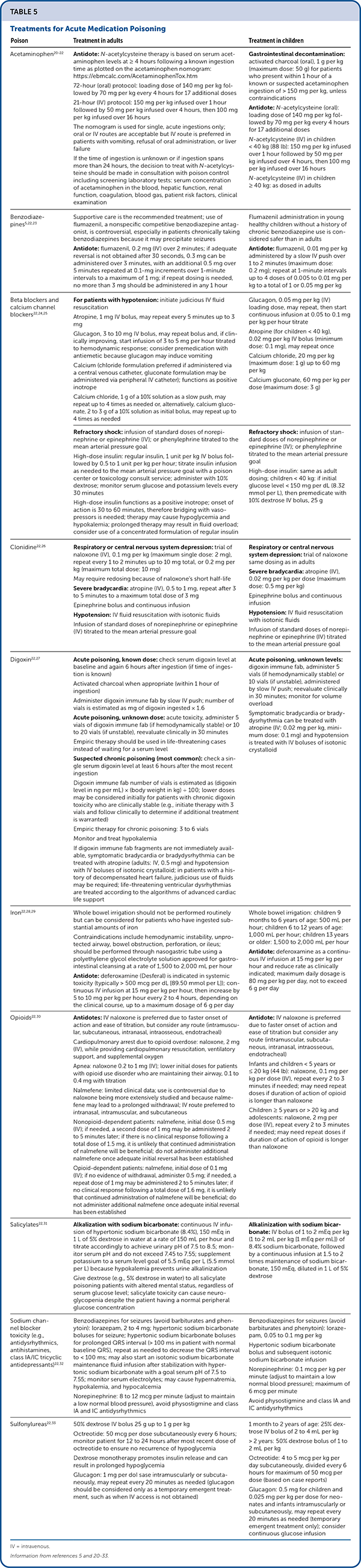
| Poison | Treatment in adults | Treatment in children |
|---|---|---|
| Acetaminophen20–22 | Antidote:N-acetylcysteine therapy is based on serum acetaminophen levels at ≥ 4 hours following a known ingestion time as plotted on the acetaminophen nomogram: https://ebmcalc.com/AcetaminophenTox.htm 72-hour (oral) protocol: loading dose of 140 mg per kg followed by 70 mg per kg every 4 hours for 17 additional doses 21-hour (IV) protocol: 150 mg per kg infused over 1 hour followed by 50 mg per kg infused over 4 hours, then 100 mg per kg infused over 16 hours The nomogram is used for single, acute ingestions only; oral or IV routes are acceptable but IV route is preferred in patients with vomiting, refusal of oral administration, or liver failure If the time of ingestion is unknown or if ingestion spans more than 24 hours, the decision to treat with N-acetylcysteine should be made in consultation with poison control including screening laboratory tests: serum concentration of acetaminophen in the blood, hepatic function, renal function, coagulation, blood gas, patient risk factors, clinical examination | Gastrointestinal decontamination: activated charcoal (oral), 1 g per kg (maximum dose: 50 g) for patients who present within 1 hour of a known or suspected acetaminophen ingestion of > 150 mg per kg, unless contraindications Antidote:N-acetylcysteine (oral): loading dose of 140 mg per kg followed by 70 mg per kg every 4 hours for 17 additional doses N-acetylcysteine (IV) in children < 40 kg (88 lb): 150 mg per kg infused over 1 hour followed by 50 mg per kg infused over 4 hours, then 100 mg per kg infused over 16 hours N-acetylcysteine (IV) in children ≥ 40 kg: as dosed in adults |
| Benzodiazepines5,22,23 | Supportive care is the recommended treatment; use of flumazenil, a nonspecific competitive benzodiazepine antagonist, is controversial, especially in patients chronically taking benzodiazepines because it may precipitate seizures Antidote: flumazenil, 0.2 mg (IV) over 2 minutes; if adequate reversal is not obtained after 30 seconds, 0.3 mg can be administered over 3 minutes, with an additional 0.5 mg over 5 minutes repeated at 0.1-mg increments over 1-minute intervals to a maximum of 1 mg; if repeat dosing is needed, no more than 3 mg should be administered in any 1 hour | Flumazenil administration in young healthy children without a history of chronic benzodiazepine use is considered safer than in adults Antidote: flumazenil, 0.01 mg per kg administered by a slow IV push over 1 to 2 minutes (maximum dose: 0.2 mg); repeat at 1-minute intervals up to 4 doses of 0.005 to 0.01 mg per kg to a total of 1 or 0.05 mg per kg |
| Beta blockers and calcium channel blockers22,24,25 | For patients with hypotension: initiate judicious IV fluid resuscitation Atropine, 1 mg IV bolus, may repeat every 5 minutes up to 3 mg Glucagon, 3 to 10 mg IV bolus, may repeat bolus and, if clinically improving, start infusion of 3 to 5 mg per hour titrated to hemodynamic response; consider premedication with antiemetic because glucagon may induce vomiting Calcium (chloride formulation preferred if administered via a central venous catheter, gluconate formulation may be administered via peripheral IV catheter); functions as positive inotrope Calcium chloride, 1 g of a 10% solution as a slow push, may repeat up to 4 times as needed or, alternatively, calcium gluconate, 2 to 3 g of a 10% solution as initial bolus, may repeat up to 4 times as needed | Glucagon, 0.05 mg per kg (IV) loading dose, may repeat, then start continuous infusion at 0.05 to 0.1 mg per kg per hour titrate Atropine (for children < 40 kg), 0.02 mg per kg IV bolus (minimum dose: 0.1 mg), may repeat once Calcium chloride, 20 mg per kg (maximum dose: 1 g) up to 60 mg per kg Calcium gluconate, 60 mg per kg per dose (maximum dose: 3 g) |
| Refractory shock: infusion of standard doses of norepinephrine or epinephrine (IV); or phenylephrine titrated to the mean arterial pressure goal High-dose insulin: regular insulin, 1 unit per kg IV bolus followed by 0.5 to 1 unit per kg per hour; titrate insulin infusion as needed to the mean arterial pressure goal with a poison center or toxicology consult service; administer with 10% dextrose; monitor serum glucose and potassium levels every 30 minutes High-dose insulin functions as a positive inotrope; onset of action is 30 to 60 minutes, therefore bridging with vasopressors is needed; therapy may cause hypoglycemia and hypokalemia; prolonged therapy may result in fluid overload; consider use of a concentrated formulation of regular insulin | Refractory shock: infusion of standard doses of norepinephrine or epinephrine (IV); or phenylephrine titrated to the mean arterial pressure goal High-dose insulin: same as adult dosing; children < 40 kg: if initial glucose level < 150 mg per dL (8.32 mmol per L), then premedicate with 10% dextrose IV bolus, 25 g | |
| Clonidine22,26 | Respiratory or central nervous system depression: trial of naloxone (IV), 0.1 mg per kg (maximum single dose: 2 mg), repeat every 1 to 2 minutes up to 10 mg total, or 0.2 mg per kg (maximum total dose: 10 mg) May require redosing because of naloxone's short half-life Severe bradycardia: atropine (IV), 0.5 to 1 mg, repeat after 3 to 5 minutes to a maximum total dose of 3 mg Epinephrine bolus and continuous infusion Hypotension: IV fluid resuscitation with isotonic fluids Infusion of standard doses of norepinephrine or epinephrine (IV) titrated to the mean arterial pressure goal | Respiratory or central nervous system depression: trial of naloxone same dosing as in adults Severe bradycardia: atropine (IV), 0.02 mg per kg per dose (maximum dose: 0.5 mg per kg) Epinephrine bolus and continuous infusion Hypotension: IV fluid resuscitation with isotonic fluids Infusion of standard doses of norepinephrine or epinephrine (IV) titrated to the mean arterial pressure goal |
| Digoxin22,27 | Acute poisoning, known dose: check serum digoxin level at baseline and again 6 hours after ingestion (if time of ingestion is known) Activated charcoal when appropriate (within 1 hour of ingestion) Administer digoxin immune fab by slow IV push; number of vials is estimated as mg of digoxin ingested × 1.6 Acute poisoning, unknown dose: acute toxicity, administer 5 vials of digoxin immune fab (if hemodynamically stable) or 10 to 20 vials (if unstable), reevaluate clinically in 30 minutes Empiric therapy should be used in life-threatening cases instead of waiting for a serum level Suspected chronic poisoning (most common): check a single serum digoxin level at least 6 hours after the most recent ingestion Digoxin immune fab number of vials is estimated as (digoxin level in ng per mL) × (body weight in kg) ÷ 100; lower doses may be considered initially for patients with chronic digoxin toxicity who are clinically stable (e.g., initiate therapy with 3 vials and follow clinically to determine if additional treatment is warranted) Empiric therapy for chronic poisoning: 3 to 6 vials Monitor and treat hypokalemia If digoxin immune fab fragments are not immediately available, symptomatic bradycardia or bradydysrhythmia can be treated with atropine (adults: IV, 0.5 mg) and hypotension with IV boluses of isotonic crystalloid; in patients with a history of decompensated heart failure, judicious use of fluids may be required; life-threatening ventricular dysrhythmias are treated according to the algorithms of advanced cardiac life support | Acute poisoning, unknown levels: digoxin immune fab, administer 5 vials (if hemodynamically stable) or 10 vials (if unstable), administered by slow IV push; reevaluate clinically in 30 minutes; monitor for volume overload Symptomatic bradycardia or bradydysrhythmia can be treated with atropine (IV; 0.02 mg per kg, minimum dose: 0.1 mg) and hypotension is treated with IV boluses of isotonic crystalloid |
| Iron22,28,29 | Whole bowel irrigation should not be performed routinely but can be considered for patients who have ingested substantial amounts of iron Contraindications include hemodynamic instability, unprotected airway, bowel obstruction, perforation, or ileus; should be performed through nasogastric tube using a polyethylene glycol electrolyte solution approved for gastrointestinal cleansing at a rate of 1,500 to 2,000 mL per hour Antidote: deferoxamine (Desferal) is indicated in systemic toxicity (typically > 500 mcg per dL [89.50 mmol per L]); continuous IV infusion at 15 mg per kg per hour, then increase by 5 to 10 mg per kg per hour every 2 to 4 hours, depending on the clinical course, up to a maximum dosage of 6 g per day | Whole bowel irrigation: children 9 months to 6 years of age: 500 mL per hour; children 6 to 12 years of age: 1,000 mL per hour; children 13 years or older: 1,500 to 2,000 mL per hour Antidote: deferoxamine as a continuous IV infusion at 15 mg per kg per hour and reduce rate as clinically indicated; maximum daily dosage is 80 mg per kg per day, not to exceed 6 g per day |
| Opioids22,30 | Antidotes: IV naloxone is preferred due to faster onset of action and ease of titration, but consider any route (intramuscular, subcutaneous, intranasal, intraosseous, endotracheal) Cardiopulmonary arrest due to opioid overdose: naloxone, 2 mg (IV), while providing cardiopulmonary resuscitation, ventilatory support, and supplemental oxygen Apnea: naloxone 0.2 to 1 mg (IV); lower initial doses for patients with opioid use disorder who are maintaining their airway, 0.1 to 0.4 mg with titration Nalmefene: limited clinical data; use is controversial due to naloxone being more extensively studied and because nalmefene may lead to a prolonged withdrawal; IV route preferred to intranasal, intramuscular, and subcutaneous Nonopioid-dependent patients: nalmefene, initial dose 0.5 mg (IV); if needed, a second dose of 1 mg may be administered 2 to 5 minutes later; if there is no clinical response following a total dose of 1.5 mg, it is unlikely that continued administration of nalmefene will be beneficial; do not administer additional nalmefene once adequate initial reversal has been established Opioid-dependent patients: nalmefene, initial dose of 0.1 mg (IV); if no evidence of withdrawal, administer 0.5 mg; if needed, a repeat dose of 1 mg may be administered 2 to 5 minutes later; if no clinical response following a total dose of 1.6 mg, it is unlikely that continued administration of nalmefene will be beneficial; do not administer additional nalmefene once adequate initial reversal has been established | Antidote: IV naloxone is preferred due to faster onset of action and ease of titration but consider any route (intramuscular, subcutaneous, intranasal, intraosseous, endotracheal) Infants and children < 5 years or ≤ 20 kg (44 lb): naloxone, 0.1 mg per kg per dose (IV), repeat every 2 to 3 minutes if needed; may need repeat doses if duration of action of opioid is longer than naloxone Children ≥ 5 years or > 20 kg and adolescents: naloxone, 2 mg per dose (IV), repeat every 2 to 3 minutes if needed; may need repeat doses if duration of action of opioid is longer than naloxone |
| Salicylates22,31 | Alkalization with sodium bicarbonate: continuous IV infusion of hypertonic sodium bicarbonate (8.4%), 150 mEq in 1 L of 5% dextrose in water at a rate of 150 mL per hour and titrate accordingly to achieve urinary pH of 7.5 to 8.5; monitor serum pH and do not exceed 7.45 to 7.55; supplement potassium to a serum level goal of 5.5 mEq per L (5.5 mmol per L) because hypokalemia prevents urine alkalinization Give dextrose (e.g., 5% dextrose in water) to all salicylate poisoning patients with altered mental status, regardless of serum glucose level; salicylate toxicity can cause neuroglycopenia despite the patient having a normal peripheral glucose concentration | Alkalinization with sodium bicarbonate: IV bolus of 1 to 2 mEq per kg (1 to 2 mL per kg [1 mEq per mL]) of 8.4% sodium bicarbonate, followed by a continuous infusion at 1.5 to 2 times maintenance of sodium bicarbonate, 150 mEq, diluted in 1 L of 5% dextrose |
| Sodium channel blocker toxicity (e.g., antidysrhythmics, antihistamines, class IA/IC tricyclic antidepressants)22,32 | Benzodiazepines for seizures (avoid barbiturates and phenytoin): lorazepam, 2 to 4 mg; hypertonic sodium bicarbonate boluses for seizure; hypertonic sodium bicarbonate boluses for prolonged QRS interval (> 100 ms in patient with normal baseline QRS), repeat as needed to decrease the QRS interval to < 100 ms; may also start an isotonic sodium bicarbonate maintenance fluid infusion after stabilization with hypertonic sodium bicarbonate with a goal serum pH of 7.5 to 7.55; monitor serum electrolytes; may cause hypernatremia, hypokalemia, and hypocalcemia Norepinephrine: 8 to 12 mcg per minute (adjust to maintain a low normal blood pressure), avoid physostigmine and class IA and IC antidysrhythmics | Benzodiazepines for seizures (avoid barbiturates and phenytoin): lorazepam, 0.05 to 0.1 mg per kg Hypertonic sodium bicarbonate bolus and subsequent isotonic sodium bicarbonate infusion Norepinephrine: 0.1 mcg per kg per minute (adjust to maintain a low normal blood pressure); maximum of 6 mcg per minute Avoid physostigmine and class IA and IC antidysrhythmics |
| Sulfonylureas22,33 | 50% dextrose IV bolus 25 g up to 1 g per kg Octreotide: 50 mcg per dose subcutaneously every 6 hours; monitor patient for 12 to 24 hours after most recent dose of octreotide to ensure no recurrence of hypoglycemia Dextrose monotherapy promotes insulin release and can result in prolonged hypoglycemia Glucagon: 1 mg per dose intramuscularly or subcutaneously, may repeat every 20 minutes as needed (glucagon should be considered only as a temporary emergent treatment, such as when IV access is not obtained) | 1 month to 2 years of age: 25% dextrose IV bolus of 2 to 4 mL per kg > 2 years: 50% dextrose bolus of 1 to 2 mL per kg Octreotide: 4 to 5 mcg per kg per day subcutaneously, divided every 6 hours for maximum of 50 mcg per dose (based on case reports) Glucagon: 0.5 mg for children and 0.025 mg per kg per dose for neonates and infants intramuscularly or subcutaneously, may repeat every 20 minutes as needed (temporary emergent treatment only); consider continuous glucose infusion |
Corporeal treatments (those that take place inside the body) include single-dose activated charcoal, multiple-dose activated charcoal, urine alkalization, and whole bowel irrigation (Table 6).28,29,32,34–37 Single-dose activated charcoal is the most frequently used form of gastrointestinal decontamination and is typically administered orally or by nasogastric tube up to one hour following ingestion of a potentially toxic amount of a medication known to be adsorbed by charcoal. Single-dose activated charcoal is contraindicated if a patient does not have an intact or protected airway34,38 (Table 734).
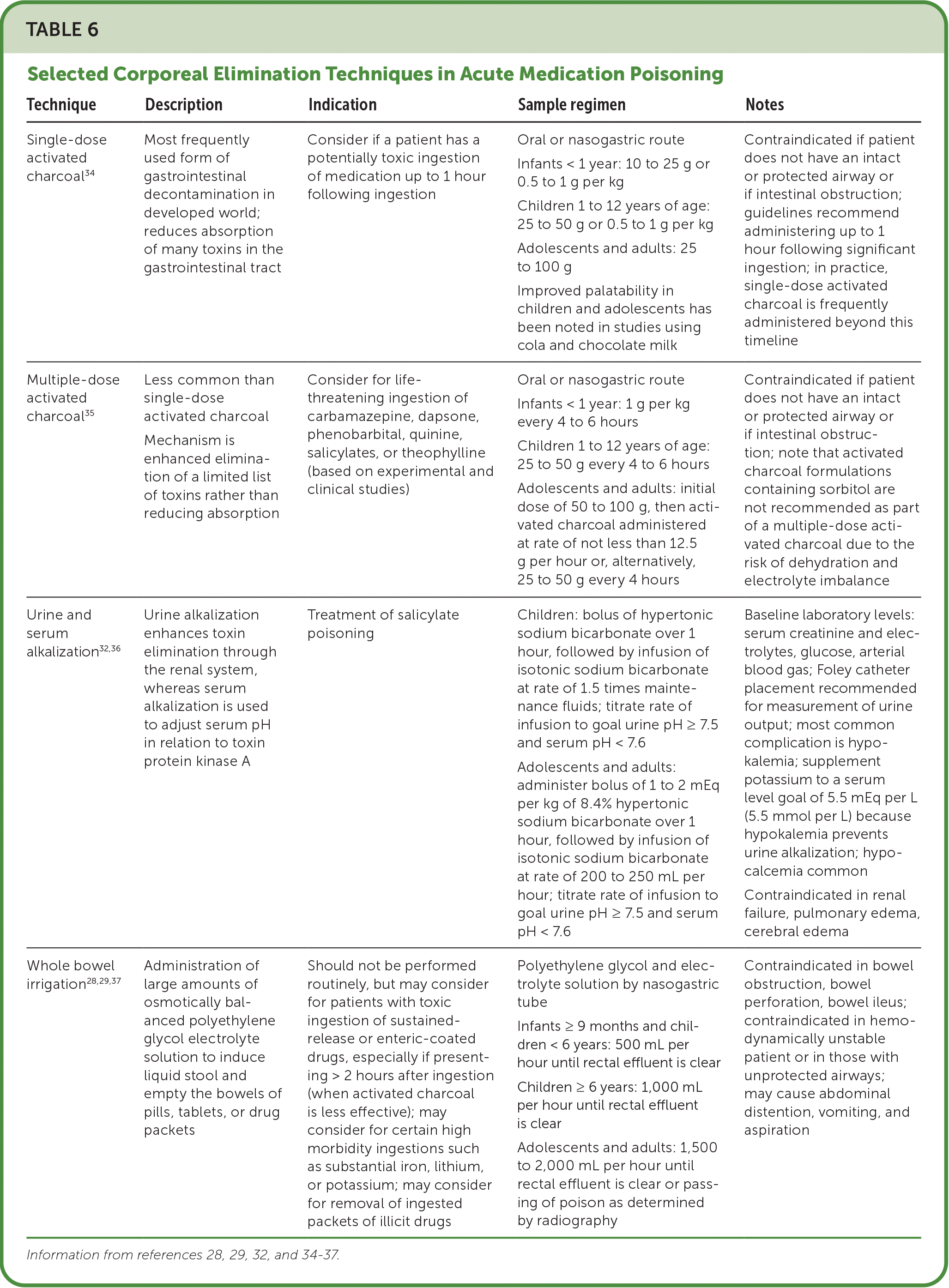
| Technique | Description | Indication | Sample regimen | Notes |
|---|---|---|---|---|
| Single-dose activated charcoal34 | Most frequently used form of gastrointestinal decontamination in developed world; reduces absorption of many toxins in the gastrointestinal tract | Consider if a patient has a potentially toxic ingestion of medication up to 1 hour following ingestion | Oral or nasogastric route Infants < 1 year: 10 to 25 g or 0.5 to 1 g per kg Children 1 to 12 years of age: 25 to 50 g or 0.5 to 1 g per kg Adolescents and adults: 25 to 100 g Improved palatability in children and adolescents has been noted in studies using cola and chocolate milk | Contraindicated if patient does not have an intact or protected airway or if intestinal obstruction; guidelines recommend administering up to 1 hour following significant ingestion; in practice, single-dose activated charcoal is frequently administered beyond this timeline |
| Multiple-dose activated charcoal35 | Less common than single-dose activated charcoal Mechanism is enhanced elimination of a limited list of toxins rather than reducing absorption | Consider for life-threatening ingestion of carbamazepine, dapsone, phenobarbital, quinine, salicylates, or theophylline (based on experimental and clinical studies) | Oral or nasogastric route Infants < 1 year: 1 g per kg every 4 to 6 hours Children 1 to 12 years of age: 25 to 50 g every 4 to 6 hours Adolescents and adults: initial dose of 50 to 100 g, then activated charcoal administered at rate of not less than 12.5 g per hour or, alternatively, 25 to 50 g every 4 hours | Contraindicated if patient does not have an intact or protected airway or if intestinal obstruction; note that activated charcoal formulations containing sorbitol are not recommended as part of a multiple-dose activated charcoal due to the risk of dehydration and electrolyte imbalance |
| Urine and serum alkalization32,36 | Urine alkalization enhances toxin elimination through the renal system, whereas serum alkalization is used to adjust serum pH in relation to toxin protein kinase A | Treatment of salicylate poisoning | Children: bolus of hypertonic sodium bicarbonate over 1 hour, followed by infusion of isotonic sodium bicarbonate at rate of 1.5 times maintenance fluids; titrate rate of infusion to goal urine pH ≥ 7.5 and serum pH < 7.6 Adolescents and adults: administer bolus of 1 to 2 mEq per kg of 8.4% hypertonic sodium bicarbonate over 1 hour, followed by infusion of isotonic sodium bicarbonate at rate of 200 to 250 mL per hour; titrate rate of infusion to goal urine pH ≥ 7.5 and serum pH < 7.6 | Baseline laboratory levels: serum creatinine and electrolytes, glucose, arterial blood gas; Foley catheter placement recommended for measurement of urine output; most common complication is hypokalemia; supplement potassium to a serum level goal of 5.5 mEq per L (5.5 mmol per L) because hypokalemia prevents urine alkalization; hypocalcemia common Contraindicated in renal failure, pulmonary edema, cerebral edema |
| Whole bowel irrigation28,29,37 | Administration of large amounts of osmotically balanced polyethylene glycol electrolyte solution to induce liquid stool and empty the bowels of pills, tablets, or drug packets | Should not be performed routinely, but may consider for patients with toxic ingestion of sustained-release or enteric-coated drugs, especially if presenting > 2 hours after ingestion (when activated charcoal is less effective); may consider for certain high morbidity ingestions such as substantial iron, lithium, or potassium; may consider for removal of ingested packets of illicit drugs | Polyethylene glycol and electrolyte solution by nasogastric tube Infants ≥ 9 months and children < 6 years: 500 mL per hour until rectal effluent is clear Children ≥ 6 years: 1,000 mL per hour until rectal effluent is clear Adolescents and adults: 1,500 to 2,000 mL per hour until rectal effluent is clear or passing of poison as determined by radiography | Contraindicated in bowel obstruction, bowel perforation, bowel ileus; contraindicated in hemodynamically unstable patient or in those with unprotected airways; may cause abdominal distention, vomiting, and aspiration |
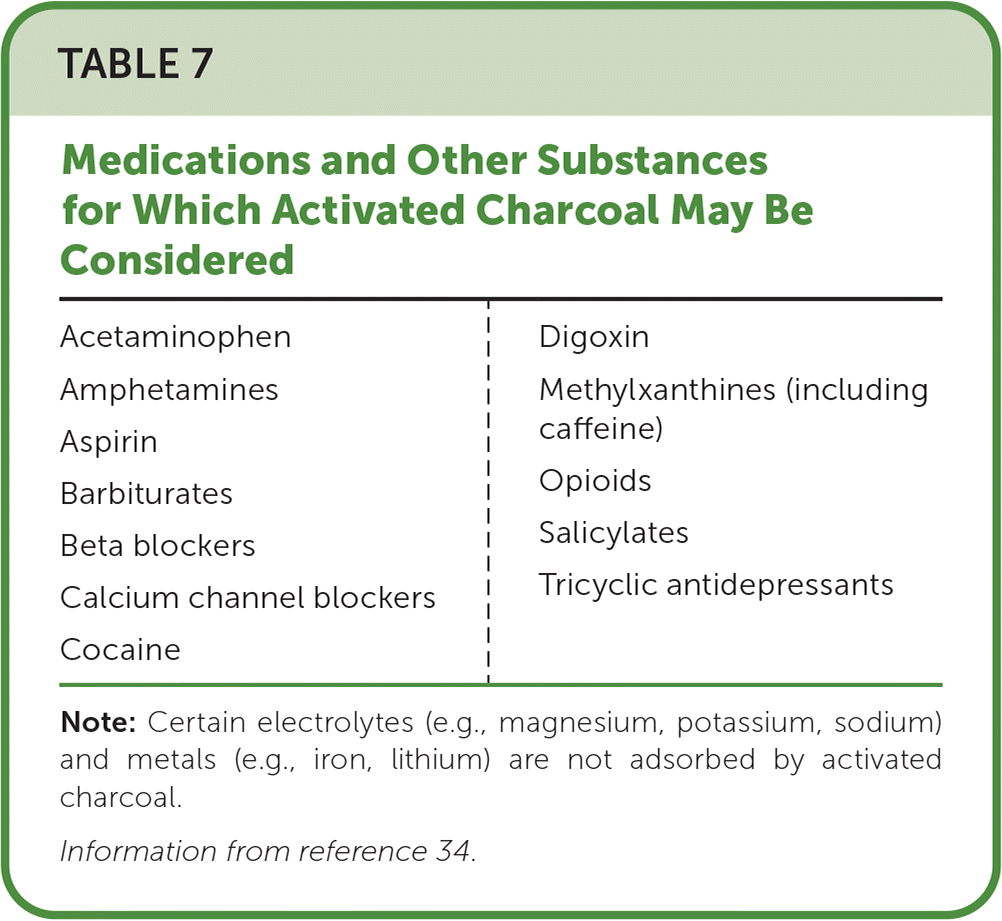
| Acetaminophen Amphetamines Aspirin Barbiturates Beta blockers Calcium channel blockers Cocaine | Digoxin Methylxanthines (including caffeine) Opioids Salicylates Tricyclic antidepressants |
No clinical data support using cathartics, including ipecac syrup, in poisoning because they have not been proven to reduce the bioavailability or toxicity of drugs and do not affect outcomes in the poisoned patient.39
Hemodialysis is a form of extracorporeal elimination for acute, severe poisoning and should be performed in consultation with a nephrologist. The ideal dialyzable substance has low molecular weight, low protein binding, and a low volume of distribution; however, dialysis can be effective for other substances that do not meet these criteria (Table 840). The Extracorporeal Treatments In Poisoning Workgroup offers recommendations for extracorporeal elimination to treat specific poisonings (https://www.extrip-workgroup.org). Mechanical circulatory support devices (e.g., extracorporeal membrane oxygenation) and intralipid therapy are treatment modalities with mixed evidence for the management of poisonings and are beyond the scope of this article.41,42
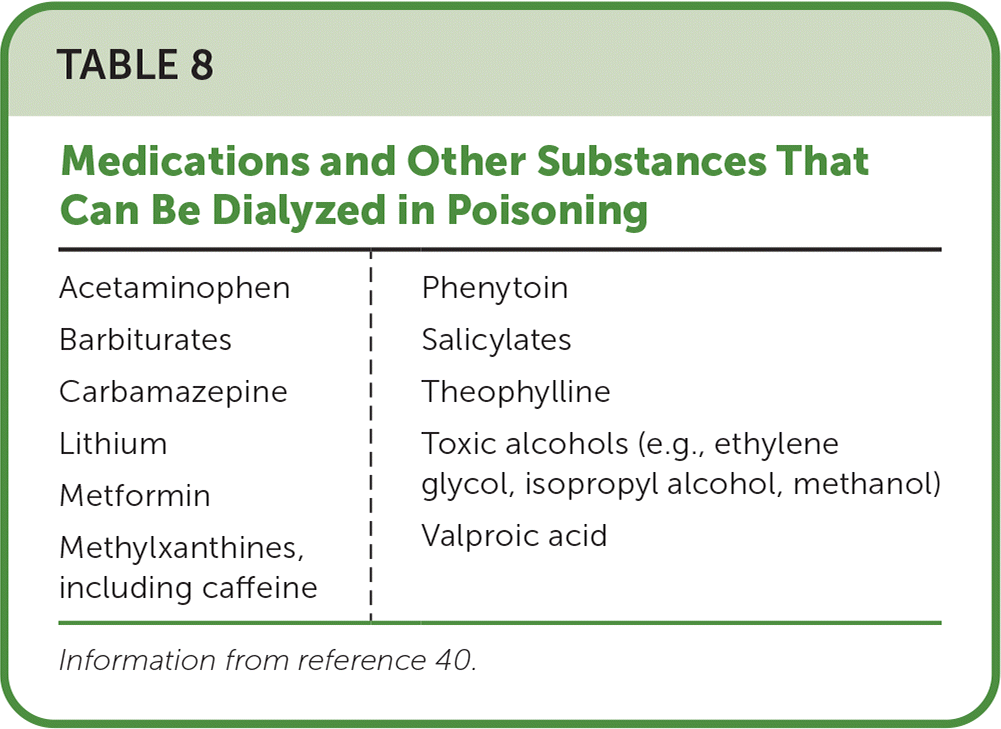
| Acetaminophen Barbiturates Carbamazepine Lithium Metformin Methylxanthines, including caffeine | Phenytoin Salicylates Theophylline Toxic alcohols (e.g., ethylene glycol, isopropyl alcohol, methanol) Valproic acid |
N-acetylcysteine is the antidote for acetaminophen toxicity and should be administered based on the ingested dose, serum acetaminophen concentration above the threshold at four hours after ingestion and later using an acetaminophen nomogram, abnormal liver biochemistry, or evidence of liver failure attributable to an overdose.20,21
DISPOSITION
Critically ill poisoned patients should be admitted to an intensive care unit or transferred to a tertiary care center. For stable patients, the length of observation should be guided by signs, symptoms, and pharmacology of the agent. Patients presenting with an intentional overdose should undergo psychiatric evaluation and may need continuous staff observation while awaiting psychiatric hospitalization. People with substance use disorder should be offered treatment and resources for harm reduction, such as naloxone and buprenorphine.43
Clinical Scenario
A 13-year-old with a history of depression presented to the emergency department after admitting to ingesting a handful (estimated 20 to 30) of 325-mg ferrous sulfate tablets one hour before arrival with suicidal intent. Vital signs and mental status were normal; weight was 45 kg (99 lb). The patient reported nausea and mild abdominal pain that resolved shortly after arrival. The workup did not find evidence of metabolic acidosis or transaminitis. Plain radiography of the abdomen showed multiple radiopaque densities in the stomach. The initial serum iron level obtained at arrival was 283 mcg per dL (50.66 μmol per L). Poison control recommended admission to the hospital for monitoring and administration of deferoxamine (Desferal) if serum iron levels rose above 500 mcg per dL (89.50 μmol per L). The treatment threshold for whole bowel irrigation and chelation is 60 mg per kg of elemental iron or 2,700 mg of elemental iron for a patient weighing 45 kg.28,29,37 Ferrous sulfate is 20% elemental iron; therefore, ingestion of 9,750 mg of ferrous sulfate (325 mg × 30 tablets) equates to 1,950 mg of elemental iron (43 mg per kg), which is below the treatment threshold. A follow-up iron level test administered six hours after ingestion peaked at 339 mcg per dL (60.68 μmol per L). The patient was discharged to an inpatient pediatric psychiatric unit after medical clearance.
This article updates a previous article on this topic by Frithsen and Simpson.44
Data Sources: A PubMed search was completed in Clinical Queries using the key terms medication poisoning, toxicity, overdose, activated charcoal, and poisoning dialysis. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. The Cochrane database and Essential Evidence Plus were also searched. Search dates: January 15, 2023, and January 21, 2024.