This is a corrected version of the article that appeared in print.
Am Fam Physician. 2024;109(2):134-142
Author disclosure: No relevant financial relationships.
The management of chronic illnesses should continue during hospitalization. Some chronic conditions require immediate intervention, whereas intensification of therapy for other conditions may be delayed until after discharge. Factors such as pain, anxiety, poor sleep hygiene, and concurrent illness can result in a transient elevation of blood pressure. Acute lowering of blood pressure in hospitalized patients who do not have target-organ damage is not recommended and may lead to harm. If treatment is needed, intravenous antihypertensive agents should be avoided. Patients with diabetes mellitus require continued management of their blood glucose while hospitalized. Noninsulin agents are typically discontinued. Blood glucose levels should be managed using basal, prandial, and/or correction insulin. During hospitalization, conservative blood glucose targets (140 to 180 mg per dL) are preferred vs. lower targets to reduce length of stay, mortality, and the risk of hypoglycemic events in critically ill patients. Alcohol use disorder is common and hospitalization for other conditions necessitates identification and management of alcohol withdrawal syndrome. The mainstay of therapy for alcohol withdrawal syndrome is benzodiazepines; however, phenobarbital is an alternative treatment option. The risk of venous thromboembolic disease is significantly increased for hospitalized patients. Venous thromboprophylaxis is recommended for all but low-risk patients. Pharmacologic prophylaxis with subcutaneous low-molecular-weight heparin is preferred; mechanical prophylaxis is an alternative for patients who are at high risk of bleeding or have contraindications to anticoagulation.
Patients who are hospitalized require continued management of their preexisting chronic medical conditions. This article reviews current evidence and recommendations for management of hypertension, diabetes mellitus, alcohol withdrawal syndrome (AWS), and venous thromboembolism (VTE) risk in hospitalized patients.
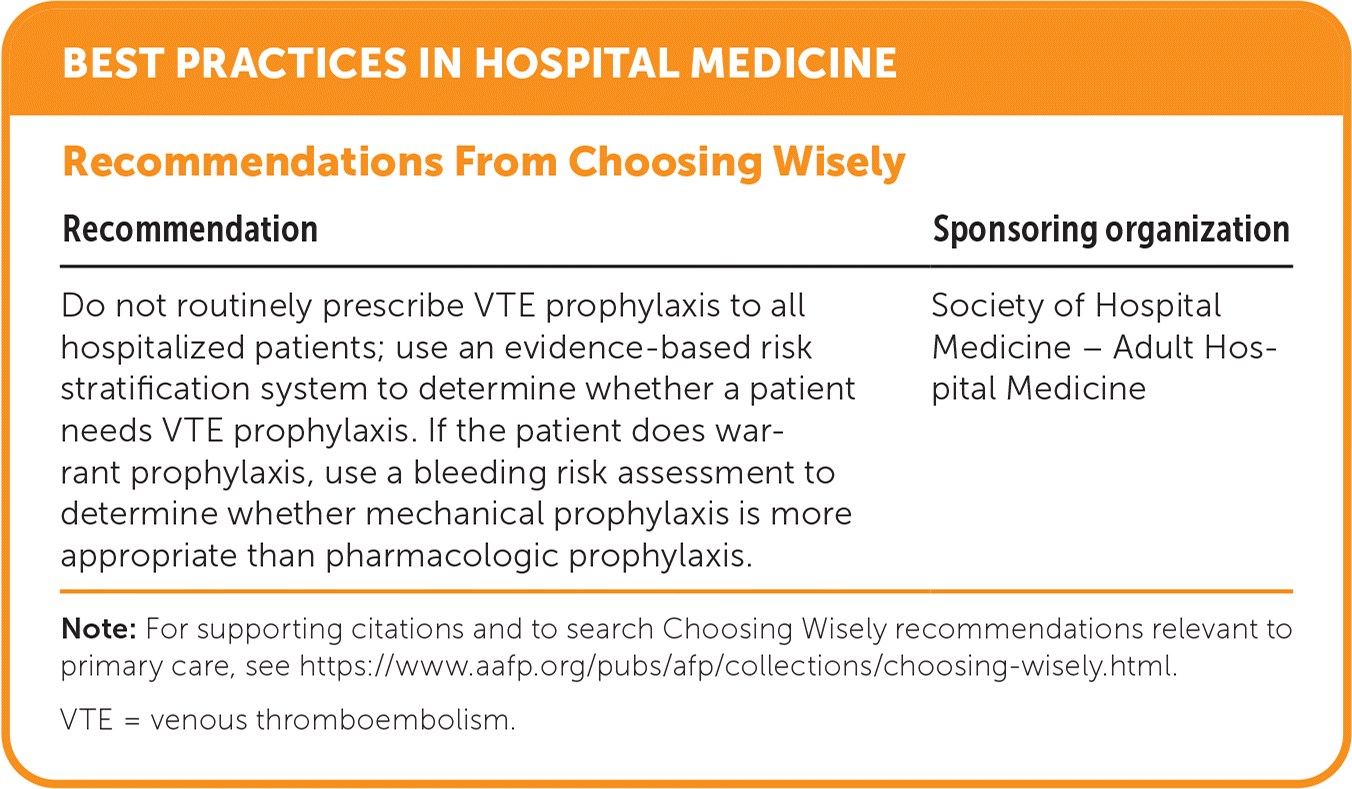
| Recommendation | Sponsoring organization |
|---|---|
| Do not routinely prescribe VTE prophylaxis to all hospitalized patients; use an evidence-based risk stratification system to determine whether a patient needs VTE prophylaxis. If the patient does warrant prophylaxis, use a bleeding risk assessment to determine whether mechanical prophylaxis is more appropriate than pharmacologic prophylaxis. | Society of Hospital Medicine – Adult Hospital Medicine |
Management of Hypertension
EPIDEMIOLOGY
The prevalence of hypertension in the inpatient setting is between 51% and 72%, based on 24-hour blood pressure monitoring.1,2 The average length of hospitalization is six days.3 Clinicians can be inconsistent with intensification of antihypertensive therapy, and 37% to 77% of patients remain hypertensive at discharge.1 Patients with hypertension are often treated with intravenous or oral medications (8%) during hospitalization or discharged with a new anti-hypertensive medication (9%). Both approaches increase the risk of harm, including acute kidney injury (10.3% vs. 7.9%; P < .001) and myocardial infarction (1.2% vs. 0.6%; P = .003).4 Blood pressure lability during hospitalization and the lack of short-term harm from higher blood pressure supports a conservative approach to hypertension during hospitalization.
Multiple trials in outpatient, inpatient, and emergency department settings have shown that treatment of severe asymptomatic hypertension (blood pressure greater than 180/110 mm Hg) has no benefit, and in some cases leads to harm.2,4–9 Despite the evidence, inpatient clinicians commonly treat patients with severe asymptomatic hypertension with oral and intravenous antihypertensive therapy. Hospital order sets routinely have preset treatment protocols when blood pressure measurements exceed 180/100 mm Hg, and in some cases, when systolic blood pressure is greater than 160 mm Hg. No studies demonstrate that patients with severe asymptomatic hypertension will progress to a hypertensive emergency.1
Hospitalized patients with elevated blood pressure often see a decrease of 20 mm Hg with subsequent measurements.4 Thirty minutes of rest is as effective as antihypertensive medications and should be first-line therapy.10 Conditions associated with transient blood pressure elevations (e.g., pain, transient kidney dysfunction, anxiety, fluid overload, agitation, nausea, poor sleep hygiene, urinary retention, nicotine or alcohol withdrawal) should be addressed.11
PHARMACOLOGIC TREATMENT
Treatment should be considered in patients who have symptoms of acute elevated blood pressure, such as headache, lightheadedness, nausea, atypical chest pain, or epistaxis. Patients with coronary artery, cerebrovascular, or chronic kidney disease, or nonoperative aortic aneurysm are considered high risk for adverse events. In these patients, pharmacologic management of sustained severe hypertension should be initiated after other causes of blood pressure elevation have been excluded. Figure 1 provides an algorithm for inpatient management of severe hypertension.11,12

Clinicians should avoid using intravenous therapy. Short-acting antihypertensive therapies (i.e., clonidine, captopril, hydralazine, labetalol, nifedipine, prazosin, and 2% topical nitroglycerin) should be used with extreme caution because they can lower blood pressure too quickly.13 Lowering blood pressure by more than 20% to 25% can result in cerebrovascular or myocardial ischemia.11 Low-risk patients with no history of hypertension and sustained severe asymptomatic hypertension do not require inpatient treatment but should have outpatient follow-up with their primary care physician.
Diabetes Management
Patients with diabetes have a threefold higher risk of hospitalization; increased length of stay; and higher rates of infection, morbidity, and mortality.14,15 Up to 25% of patients with no known diabetes will develop hyperglycemia (e.g., stress hyperglycemia, undiagnosed diabetes) during hospitalization. 16 Obtaining a blood glucose level for all patients at admission is recommended. An A1C level should be obtained for patients with diabetes who have not had one in the past three months and those with a blood glucose level greater than 140 mg per dL (7.77 mmol per L) at admission. The 2022 Endocrine Society clinical practice guidelines recommend using continuous blood glucose monitoring in patients with diabetes who require insulin and are hospitalized for noncritical illnesses, but some hospitals may not have this capability.17
The NICE-SUGAR trial found that mortality rates were increased in critically ill patients who had intensive blood glucose control (81 to 108 mg per dL [4.5 to 5.99 mmol per L]) compared with conventional blood glucose control (140 to 180 mg per dL [7.77 to 9.99 mmol per L]).18 The benefits and harms of intensive glycemic control in patients hospitalized for noncritical illnesses are unknown. Surgical patients have significantly less complications with basal-bolus insulin compared with correction insulin alone.14 The American Diabetes Association recommends insulin if blood glucose is 180 mg per dL or greater. They acknowledge that lower goals (110 to 140 mg per dL [6.11 to 7.77 mmol per L]) can be used if there is no increased risk of hypoglycemia, such as in patients after heart surgery or those who are critically ill after surgery.19 The Endocrine Society recommends a broader goal of 100 to 180 mg per dL (5.55 to 9.99 mmol per L).17
MEDICATION TREATMENT
A basal-bolus insulin regimen is recommended for treatment of hyperglycemia in hospitalized patients.20 Basal-bolus insulin is a combination of: (1) basal insulin, (2) prandial insulin (i.e., bolus premeal insulin), and (3) correction insulin.14,16,17,19 The use of basal-bolus insulin can require four or five separate injections using two different insulin formulations and is dependent on nutritional intake. Sliding scale insulin alone is not recommended except in patients with well-controlled, mild type 2 diabetes who are not on insulin.21,22 Sliding scale insulin is a reactive approach to hyperglycemia and not anticipatory glycemic management. Sliding scale insulin used with basal and prandial insulin therapies is often referred to as correction insulin. Table 1 provides definitions for these insulin terms. The amount of correction insulin required will guide titration of basal and prandial regimens.
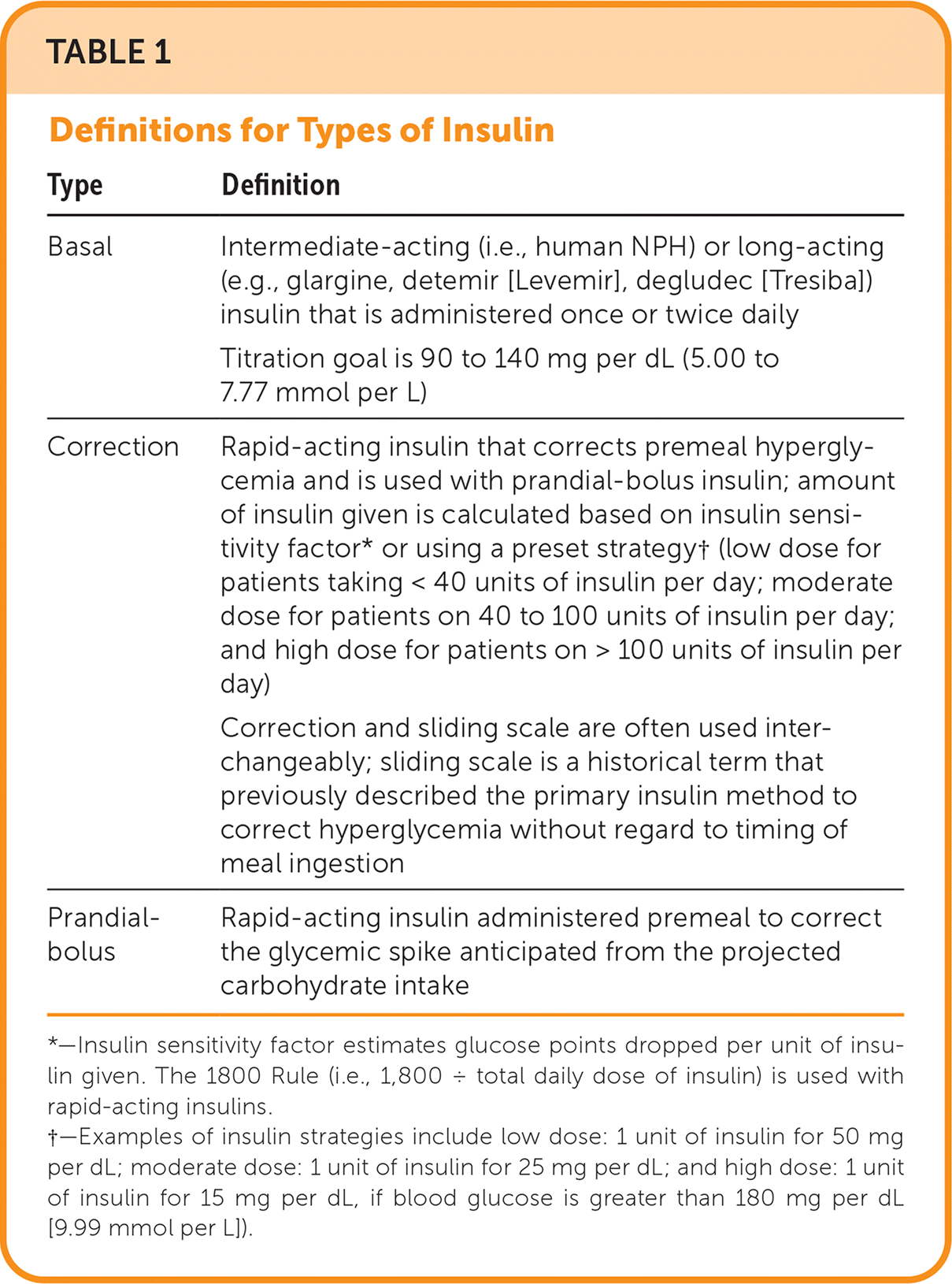
| Type | Definition |
|---|---|
| Basal | Intermediate-acting (i.e., human NPH) or long-acting (e.g., glargine, detemir [Levemir], degludec [Tresiba]) insulin that is administered once or twice daily Titration goal is 90 to 140 mg per dL (5.00 to 7.77 mmol per L) |
| Correction | Rapid-acting insulin that corrects premeal hyperglycemia and is used with prandial-bolus insulin; amount of insulin given is calculated based on insulin sensitivity factor* or using a preset strategy† (low dose for patients taking < 40 units of insulin per day; moderate dose for patients on 40 to 100 units of insulin per day; and high dose for patients on > 100 units of insulin per day) Correction and sliding scale are often used interchangeably; sliding scale is a historical term that previously described the primary insulin method to correct hyperglycemia without regard to timing of meal ingestion |
| Prandial-bolus | Rapid-acting insulin administered premeal to correct the glycemic spike anticipated from the projected carbohydrate intake |
Patients with well-controlled diabetes who do not require insulin and are able to eat, have a short anticipated hospitalization, and are not critically ill may continue oral hypoglycemic agents; noninsulin agents should be discontinued in other patients.14,16,17,19 Oral hypoglycemic agents are more likely to be stopped during longer admissions. These include metformin (should be temporarily withheld for iodinated contrast agents; risk of lactic acidosis), sulfonylureas (unpredictable hypoglycemia), meglitinides (increased risk of cardiovascular events), and thiazolidinediones (risk of peripheral edema; should be avoided in heart failure). Recent studies have shown that glucagon-like peptide-1 (GLP-1) agonists and dipeptidyl-peptidase-4 (DPP-4) inhibitors for mild hyperglycemia have outcomes similar to basal-bolus insulin.16,23 Use of GLP-1 agonists or DPP-4 inhibitors with basal insulin has similar glycemic control and less hypoglycemia compared with basal-bolus insulin alone.22 Sodium-glucose cotransporter-2 inhibitors are used for glycemic control, particularly in patients with heart failure. An approach to glycemic management in inpatients with diabetes is outlined in Table 2.14,15,23
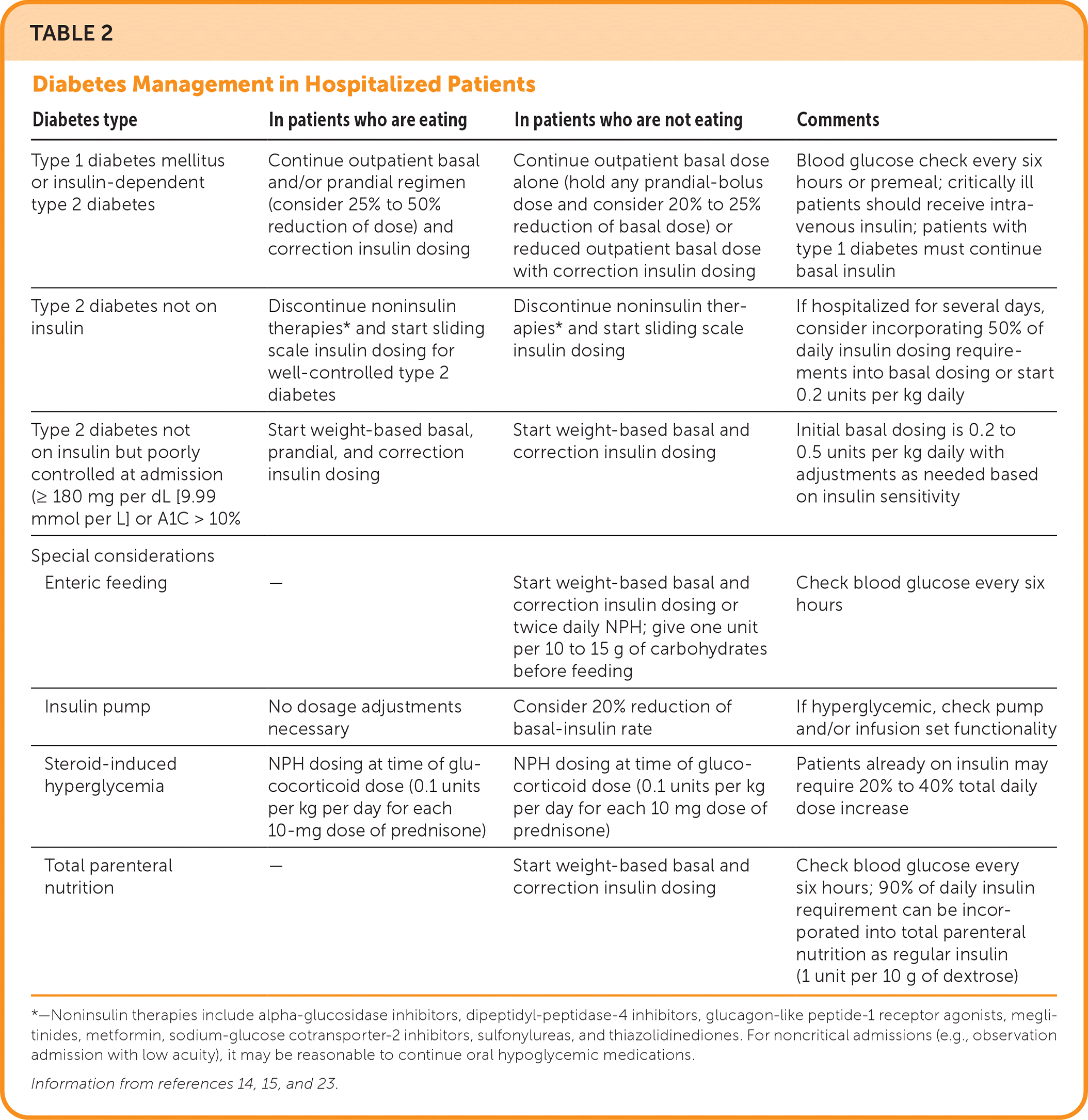
| Diabetes type | In patients who are eating | In patients who are not eating | Comments |
|---|---|---|---|
| Type 1 diabetes mellitus or insulin-dependent type 2 diabetes | Continue outpatient basal and/or prandial regimen (consider 25% to 50% reduction of dose) and correction insulin dosing | Continue outpatient basal dose alone (hold any prandial-bolus dose and consider 20% to 25% reduction of basal dose) or reduced outpatient basal dose with correction insulin dosing | Blood glucose check every six hours or premeal; critically ill patients should receive intravenous insulin; patients with type 1 diabetes must continue basal insulin |
| Type 2 diabetes not on insulin | Discontinue noninsulin therapies* and start sliding scale insulin dosing for well-controlled type 2 diabetes | Discontinue noninsulin therapies* and start sliding scale insulin dosing | If hospitalized for several days, consider incorporating 50% of daily insulin dosing requirements into basal dosing or start 0.2 units per kg daily |
| Type 2 diabetes not on insulin but poorly controlled at admission (≥ 180 mg per dL [9.99 mmol per L] or A1C > 10% | Start weight-based basal, prandial, and correction insulin dosing | Start weight-based basal and correction insulin dosing | Initial basal dosing is 0.2 to 0.5 units per kg daily with adjustments as needed based on insulin sensitivity |
| Special considerations | |||
| Enteric feeding | — | Start weight-based basal and correction insulin dosing or twice daily NPH; give one unit per 10 to 15 g of carbohydrates before feeding | Check blood glucose every six hours |
| Insulin pump | No dosage adjustments necessary | Consider 20% reduction of basal-insulin rate | If hyperglycemic, check pump and/or infusion set functionality |
| Steroid-induced hyperglycemia | NPH dosing at time of glucocorticoid dose (0.1 units per kg per day for each 10-mg dose of prednisone) | NPH dosing at time of glucocorticoid dose (0.1 units per kg per day for each 10 mg dose of prednisone) | Patients already on insulin may require 20% to 40% total daily dose increase |
| Total parenteral nutrition | — | Start weight-based basal and correction insulin dosing | Check blood glucose every six hours; 90% of daily insulin requirement can be incorporated into total parenteral nutrition as regular insulin (1 unit per 10 g of dextrose) |
Patients with an insulin pump can continue using it during hospitalization if they are not critically ill and do not have an impaired level of consciousness or poor blood glucose control. It is recommended that hospitals have personnel with expertise in insulin pumps. Patients whose insulin pumps are discontinued (i.e., due to critical illness, equipment failure, lack of supplies, altered mental status, or the need for magnetic resonance imaging) should be started on subcutaneous or intravenous insulin within 30 minutes. Patients with inadequately controlled diabetes or an elevated A1C level should have intensification of therapy and education by a certified diabetic educator before discharge. These interventions reduce hypo- and hyperglycemic events, surgical infection rates, and length of stay.24
Management of Alcohol Use Disorder and Withdrawal Syndrome
RISK ASSESSMENT
AWS is a life-threatening condition with a 3% mortality rate. The condition develops in 2% to 7% of patients with heavy alcohol use who are admitted to general medical units.25 Mortality is as high as 23% in patients hospitalized for AWS.26 Of hospitalized patients, 33% meet the criteria for alcohol dependence.27 Symptoms of AWS begin six hours after the patient's last drink and range from mild to severe (Table 3).28–30 An alcohol use history should be obtained for all hospitalized patients and include complications associated with past alcohol withdrawal, such as seizures or delirium tremens.27–29 Most patients will develop only mild withdrawal symptoms; however, 25% will develop more severe AWS.26
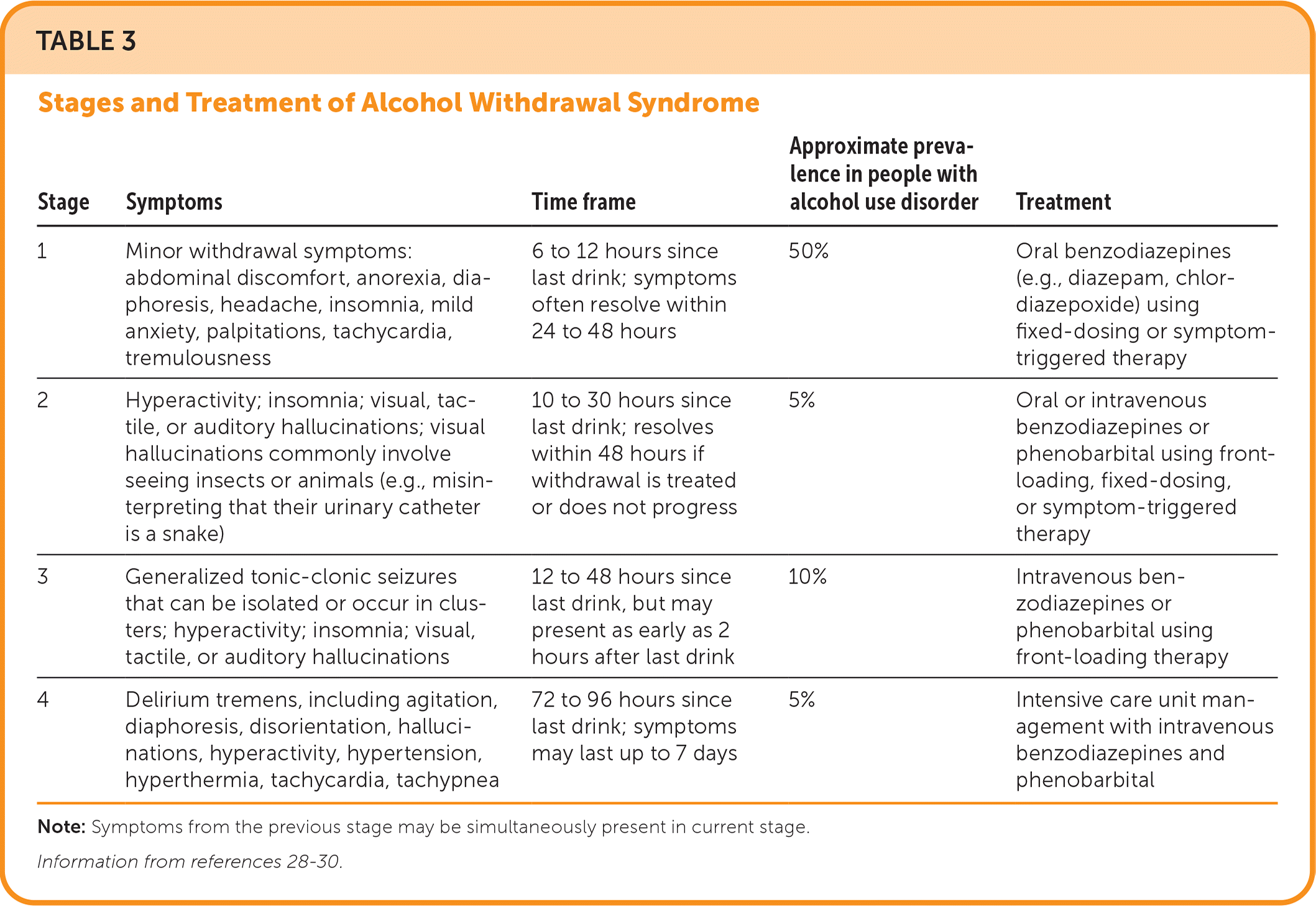
| Stage | Symptoms | Time frame | Approximate prevalence in people with alcohol use disorder | Treatment |
|---|---|---|---|---|
| 1 | Minor withdrawal symptoms: abdominal discomfort, anorexia, diaphoresis, headache, insomnia, mild anxiety, palpitations, tachycardia, tremulousness | 6 to 12 hours since last drink; symptoms often resolve within 24 to 48 hours | 50% | Oral benzodiazepines (e.g., diazepam, chlordiazepoxide) using fixed-dosing or symptom-triggered therapy |
| 2 | Hyperactivity; insomnia; visual, tactile, or auditory hallucinations; visual hallucinations commonly involve seeing insects or animals (e.g., misinterpreting that their urinary catheter is a snake) | 10 to 30 hours since last drink; resolves within 48 hours if withdrawal is treated or does not progress | 5% | Oral or intravenous benzodiazepines or phenobarbital using front-loading, fixed-dosing, or symptom-triggered therapy |
| 3 | Generalized tonic-clonic seizures that can be isolated or occur in clusters; hyperactivity; insomnia; visual, tactile, or auditory hallucinations | 12 to 48 hours since last drink, but may present as early as 2 hours after last drink | 10% | Intravenous benzodiazepines or phenobarbital using front-loading therapy |
| 4 | Delirium tremens, including agitation, diaphoresis, disorientation, hallucinations, hyperactivity, hypertension, hyperthermia, tachycardia, tachypnea | 72 to 96 hours since last drink; symptoms may last up to 7 days | 5% | Intensive care unit management with intravenous benzodiazepines and phenobarbital |
Patients with alcohol use disorder who are at risk of AWS require regular assessment using the Clinical Institute Withdrawal Assessment for Alcohol, Revised (CIWA-Ar; https://www.mdcalc.com/calc/1736/ciwa-ar-alcohol-withdrawal) or the Prediction of Alcohol Withdrawal Severity Scale (PAWSS; https://www.mdcalc.com/calc/10102/prediction-alcohol-withdrawal-severity-scale). A CIWA-Ar score of less than 8 or a PAWSS score of less than 4 indicates mild AWS, a CIWA-Ar score between 8 and 15 indicates moderate AWS, and a CIWA-Ar score of 15 or more or a PAWSS score of 4 or more is associated with severe AWS.26,31 An assessment should be performed at least every four to eight hours but may be needed hourly to assess response to medication.
Electrolytes (e.g., potassium, magnesium, phosphate), fluids, and nutritional deficiencies should be corrected as needed. Administration of thiamine and glucose are recommended to prevent Wernicke encephalopathy.32 Critical care may be needed for patients with past complications, such as delirium tremens or withdrawal seizures, frequent sedative dosing, and severe medical complications associated with alcohol use disorder (e.g., gastrointestinal bleeding, severe pancreatitis, marked electrolyte abnormalities, severe hepatic dysfunction, sepsis).
PHARMACOLOGIC TREATMENT
The American Society of Addiction Medicine recommends the use of short-acting (e.g., lorazepam, oxazepam) or long-acting (e.g., diazepam, chlordiazepoxide) benzodiazepines as first-line treatment for AWS.32 Symptom-triggered dosing is preferred because it reduces length of stay and total benzodiazepine use. Patients at higher risk of AWS may benefit from front loading or a fixed-dose taper.33 Mild AWS can be treated with a four-day, fixed taper (i.e., diazepam, 10 mg every six hours on day 1, every eight hours on day 2, every 12 hours on day 3, and at night on day 4) or symptom-triggered dosing based on CIWA-Ar score. Moderate to severe AWS often requires intravenous loading doses (i.e., diazepam, 5 to 10 mg every 10 to 15 minutes, or lorazepam, 2 to 4 mg every 10 to 15 minutes) to achieve adequate sedation.34 Conversion to oral dosing is recommended after loading with fixed or symptom-triggered dosing. Benzodiazepines are associated with hepatic encephalopathy, excessive sedation, and respiratory depression, particularly in patients with liver impairment. Lorazepam and oxazepam have a shorter half-life and are preferred in patients with advanced liver disease or acute alcoholic hepatitis.
Phenobarbital is another treatment option that can be used as an adjunct to benzodiazepines or as monotherapy 35–40 (eFigure A). Phenobarbital has a rapid onset, a long half-life, wide therapeutic range, and no sedative effects at moderate doses. When using phenobarbital, the Richmond Agitation-Sedation Scale (https://www.mdcalc.com/calc/1872/richmond-agitation-sedation-scale-rass) offers a simple scoring system that requires only observation of the patient.

A 2023 systematic review (n = 11 trials) compared pheno-barbital with benzodiazepines. The evidence demonstrates safety, effectiveness, and noninferiority of phenobarbital alone or with benzodiazepines for AWS.41 There was no significant difference in length of stay, incidence of intubation, and the need for hospitalization (when phenobarbital was used in the emergency department).42–44 Anticonvulsants such as carbamazepine, gabapentin, and valproic acid have also been used to treat mild AWS; however, there is no evidence of their effectiveness to treat severe AWS or delirium tremens.45 Dexmedetomidine is used in the intensive care unit as an adjunct treatment for resistant alcohol withdrawal but does not prevent seizures or delirium tremens.32
Patients with alcohol use disorder should be referred to an alcohol treatment program before discharge. Naltrexone (Revia) and acamprosate increase the likelihood of abstinence.46 Naltrexone can be administered orally, once daily, or intramuscularly, every four weeks. Significantly elevated liver function enzymes (three to five times or more above normal range) and active opioid use limit the use of naltrexone. Acamprosate (three times daily) is well tolerated and ideal for patients with preexisting liver disease or those taking opioids.
Venous Thromboembolism Prevention
VTE disease is a common cause of hospital-acquired morbidity and mortality. The overall incidence of VTE in noncritically ill hospitalized patients is approximately 1.2%.47 Appropriate VTE prophylaxis reduces morbidity and the overall risk of developing deep venous thrombosis or pulmonary embolism.48 [corrected] Several prediction scores are available (e.g., Padua Prediction Score, the IMPROVE risk score, GENEVA risk score; https://www.mdcalc.com) to assess risk of VTE. Except for those at low-risk, all patients should receive prophylaxis, preferably with low-molecular-weight heparin, to reduce the risk of VTE disease.48–50 Patients with respiratory failure, acute infection, active cancer, acute limb paralysis or immobility, acquired or inherited thrombophilia, autoimmune or inflammatory conditions, a history of heart failure or VTE, or kidney failure; who are 60 years and older; or who have been admitted to the intensive care unit require VTE prophylaxis.49
Mechanical VTE prophylaxis (i.e., intermittent pneumatic compression devices) is recommended in patients at high risk of bleeding (e.g., IMPROVE Bleeding Risk Assessment Model) or who have contraindications to anticoagulation (e.g., active duodenal ulcer, bleeding within three months of admission, intracranial hemorrhage, scheduled invasive procedure, platelet count less than 50 × 103 cells per μL [50 × 109 cells per L]).49 Patients should be reevaluated daily to determine if pharmacologic VTE prophylaxis can be initiated. Combination pharmacologic and mechanical VTE prophylaxis has not been studied in hospitalized patients and is not recommended. There is an increased risk of major bleeding in patients with trauma and stroke with combined VTE prophylaxis compared with pharmacologic VTE prophylaxis alone.49 Aspirin and warfarin should not be used for VTE prophylaxis in hospitalized patients. A 2023 study demonstrated that 81 mg of aspirin, twice daily, was noninferior to low-molecular-weight heparin, 30 mg subcutaneously, twice daily, in patients with surgically repaired extremity or pelvic fractures in the prevention of VTE.51 Patients currently taking oral anticoagulation for other indications (e.g., active VTE treatment, atrial fibrillation, prosthetic heart valve, left ventricular thrombus) do not require additional prophylaxis while hospitalized.52 Patients with total joint arthroplasty (e.g., knee, hip), significant paralysis from stroke, or mechanical ventilation should receive extended prophylaxis. Table 4 lists options for pharmacologic prophylaxis for VTE.50,53–56
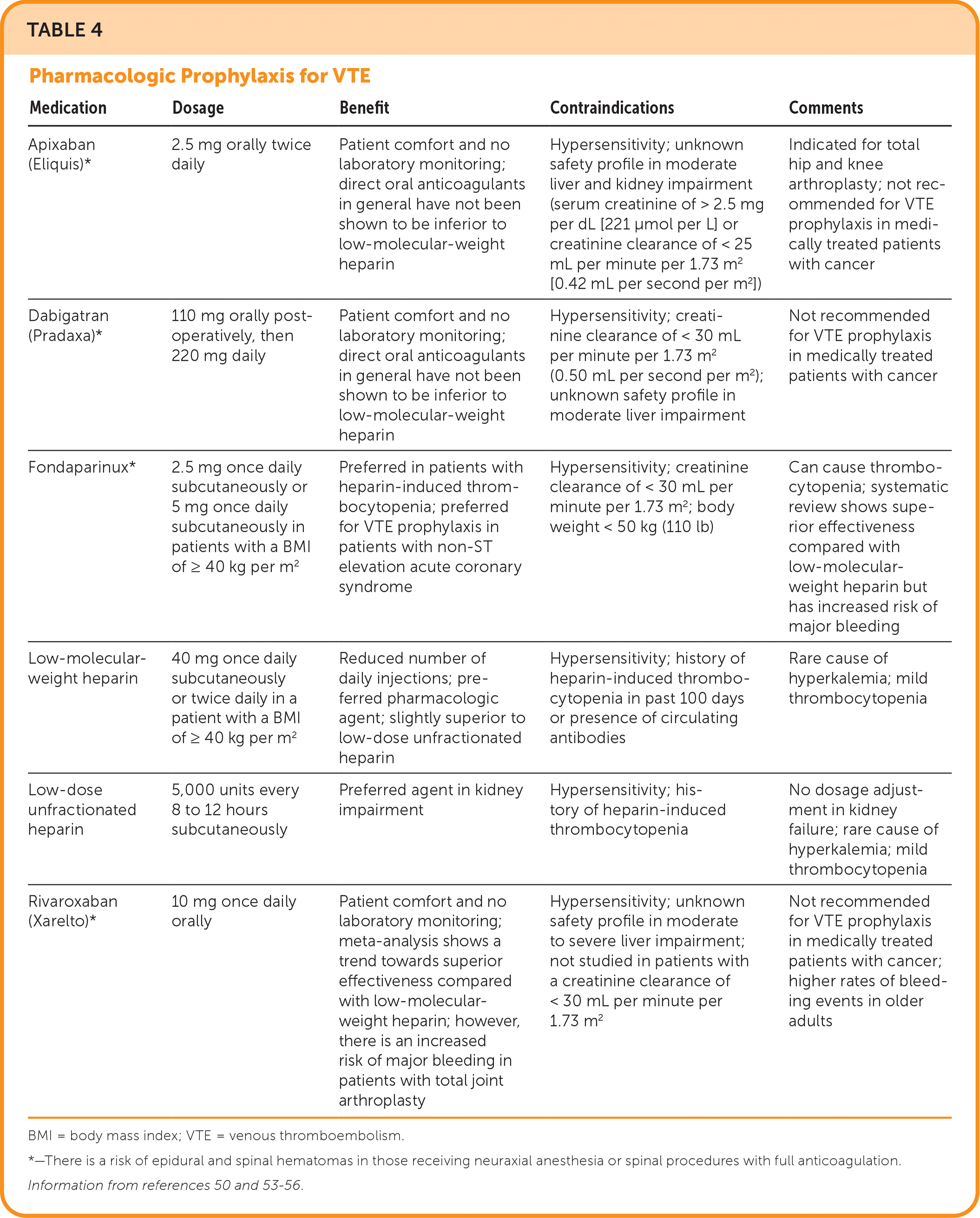
| Medication | Dosage | Benefit | Contraindications | Comments |
|---|---|---|---|---|
| Apixaban (Eliquis)* | 2.5 mg orally twice daily | Patient comfort and no laboratory monitoring; direct oral anticoagulants in general have not been shown to be inferior to low-molecular-weight heparin | Hypersensitivity; unknown safety profile in moderate liver and kidney impairment (serum creatinine of > 2.5 mg per dL [221 μmol per L] or creatinine clearance of < 25 mL per minute per 1.73 m2 [0.42 mL per second per m2]) | Indicated for total hip and knee arthroplasty; not recommended for VTE prophylaxis in medically treated patients with cancer |
| Dabigatran (Pradaxa)* | 110 mg orally postoperatively, then 220 mg daily | Patient comfort and no laboratory monitoring; direct oral anticoagulants in general have not been shown to be inferior to low-molecular-weight heparin | Hypersensitivity; creatinine clearance of < 30 mL per minute per 1.73 m2 (0.50 mL per second per m2); unknown safety profile in moderate liver impairment | Not recommended for VTE prophylaxis in medically treated patients with cancer |
| Fondaparinux* | 2.5 mg once daily subcutaneously or 5 mg once daily subcutaneously in patients with a BMI of ≥ 40 kg per m2 | Preferred in patients with heparin-induced thrombocytopenia; preferred for VTE prophylaxis in patients with non-ST elevation acute coronary syndrome | Hypersensitivity; creatinine clearance of < 30 mL per minute per 1.73 m2; body weight < 50 kg (110 lb) | Can cause thrombocytopenia; systematic review shows superior effectiveness compared with low-molecular-weight heparin but has increased risk of major bleeding |
| Low-molecular-weight heparin | 40 mg once daily subcutaneously or twice daily in a patient with a BMI of ≥ 40 kg per m2 | Reduced number of daily injections; preferred pharmacologic agent; slightly superior to low-dose unfractionated heparin | Hypersensitivity; history of heparin-induced thrombocytopenia in past 100 days or presence of circulating antibodies | Rare cause of hyperkalemia; mild thrombocytopenia |
| Low-dose unfractionated heparin | 5,000 units every 8 to 12 hours subcutaneously | Preferred agent in kidney impairment | Hypersensitivity; history of heparin-induced thrombocytopenia | No dosage adjustment in kidney failure; rare cause of hyperkalemia; mild thrombocytopenia |
| Rivaroxaban (Xarelto)* | 10 mg once daily orally | Patient comfort and no laboratory monitoring; meta-analysis shows a trend towards superior effectiveness compared with low-molecular-weight heparin; however, there is an increased risk of major bleeding in patients with total joint arthroplasty | Hypersensitivity; unknown safety profile in moderate to severe liver impairment; not studied in patients with a creatinine clearance of < 30 mL per minute per 1.73 m2 | Not recommended for VTE prophylaxis in medically treated patients with cancer; higher rates of bleeding events in older adults |
Data Sources: A search was performed in PubMed, UpToDate, Society of Hospital Medicine, American Diabetes Association, Endocrine Society clinical practice guideline, American Society of Addiction Medicine, American Psychiatric Association practice guideline, and American Society of Hematology. Key words included severe asymptomatic hypertension, hypertensive urgency, hypertensive emergency, emergency department, practice guidelines, inpatient, hospitalization, thromboembolism, prophylaxis, critically ill patients, medical adults, pharmacologic thromboprophylaxis, graduated compressing devices, direct oral anticoagulant, heparin, low-molecular-weight heparins, diabetes, glucose, diabetes type 1, diabetes type 2, sliding scale insulin, hyperglycemia, hospital standards, alcohol withdrawal syndrome, benzodiazepines, phenobarbital, and refractory alcohol withdrawal. Search dates: December 2022, and January, April, and November 2023.
The views expressed herein are those of the authors and do not necessarily reflect the official policy of the U.S. Department of the Army, U.S. Department of Defense, or U.S. government.