
Am Fam Physician. 2021;103(8):473-480
Related letter to the Editor: Risks of and Indications for Mifepristone for Medication Abortion
Related letter to the Editor: More Study Needed of Preferred Regimens for Medication Abortion Beyond 70 Days
Author disclosure: No relevant financial affiliations.
Medication regimens using mifepristone and misoprostol are safe and effective for outpatient treatment of early pregnancy loss for up to 84 days' gestation and for medication abortion up to 77 days' gestation. Gestational age is determined using ultrasonography or menstrual history. Ultrasonography is needed when gestational dating cannot be confirmed using clinical data alone or when there are risk factors for ectopic pregnancy. The most effective regimens for medication management of early pregnancy loss and medication abortion include 200 mg of oral mifepristone (a progesterone receptor antagonist) followed by 800 mcg of misoprostol (a prostaglandin E1analogue) administered buccally or vaginally. Cramping and bleeding are expected effects of the medications, with bleeding lasting an average of nine to 16 days. The adverse effects of misoprostol (e.g., low-grade fever, gastrointestinal symptoms) can be managed with nonsteroidal anti-inflammatory drugs or antiemetics. Ongoing pregnancy, infection, hemorrhage, undiagnosed ectopic pregnancy, and the need for unplanned uterine aspiration are rare complications. Clinical history, combined with serial quantitative beta human chorionic gonadotropin levels, urine pregnancy testing, or ultrasonography, is used to establish complete passage of the pregnancy tissue.
Medication management of early pregnancy loss and medication abortion has become increasingly common since the U.S. Food and Drug Administration (FDA) approval of mifepristone (Mifeprex) in 2000. Medication abortion now accounts for 60% of all abortions completed before 10 weeks' gestation.1 The most effective medication regimens combine mifepristone, a progesterone receptor antagonist that causes decidual necrosis and uterine contractions, and misoprostol (Cytotec), a prostaglandin E1 analogue that causes cervical ripening and uterine contractions. These regimens are safe and acceptable to patients and can be prescribed by primary care clinicians in the outpatient setting.2–4 Primary care clinicians are uniquely positioned to counsel patients and provide access to medications, with their wide geographic distribution, skills in shared decision-making, and longitudinal relationships with patients; however, only 1% of abortions currently occur in clinicians' offices.1

Determining Eligibility
Before prescribing mifepristone and misoprostol, clinicians should determine gestational age, evaluate for contraindications, provide patient-centered counseling on management options, and assess the need for laboratory testing.
Regimens using mifepristone and misoprostol are effective up to 84 days' gestation for early pregnancy loss,2,3 and up to 77 days' gestation for medication abortion.5–8 Ultrasonography is indicated to establish the diagnosis and confirm gestational dating before using medications for early pregnancy loss. Ultrasonography, if needed, or menstrual dating can establish that gestational age is less than 77 days before a medication abortion is provided.9–11 Ultrasonography should be performed in patients at risk of ectopic pregnancy or if gestational age cannot be confirmed using clinical data alone (Table 1).9–11

| Increased risk of ectopic pregnancy |
| Adnexal mass or tenderness on examination |
| History of ectopic pregnancy |
| History of treatment for pelvic inflammatory disease |
| History of tubal surgery, including sterilization |
| Pregnancy with intrauterine device in place |
| Vaginal bleeding or unilateral pelvic pain |
| Unable to confirm gestational age less than 11 weeks |
| Hormonal contraceptive use within the past two months |
| Last menstrual period more than 10 weeks ago |
| Unsure date of last menstrual period |
| Uterine size/date discrepancy on bimanual examination |
There are few contraindications to using mifepristone and misoprostol12 (Table 24,12,13). Medication management research has excluded patients with severe hepatic, renal, respiratory, or cardiovascular disease, or with hemoglobin levels of less than 10 g per dL (100 g per L). Laboratory testing should be considered for patients with symptoms of or at risk of anemia or sexually transmitted infections. An initial quantitative beta human chorionic gonadotropin (β-hCG) level is needed if serial β-hCG will be used to confirm completed abortion. The standard of care has been to administer RhO(D) immune globulin (Rhogam) to all patients who are Rh-negative and who are undergoing early pregnancy loss or abortion.14 However, according to preliminary research findings, the risk of alloimmunization in early gestation may be negligible.15 If future research confirms this finding, testing for Rh status may not be indicated when prescribing mifepristone and misoprostol in the first trimester.16
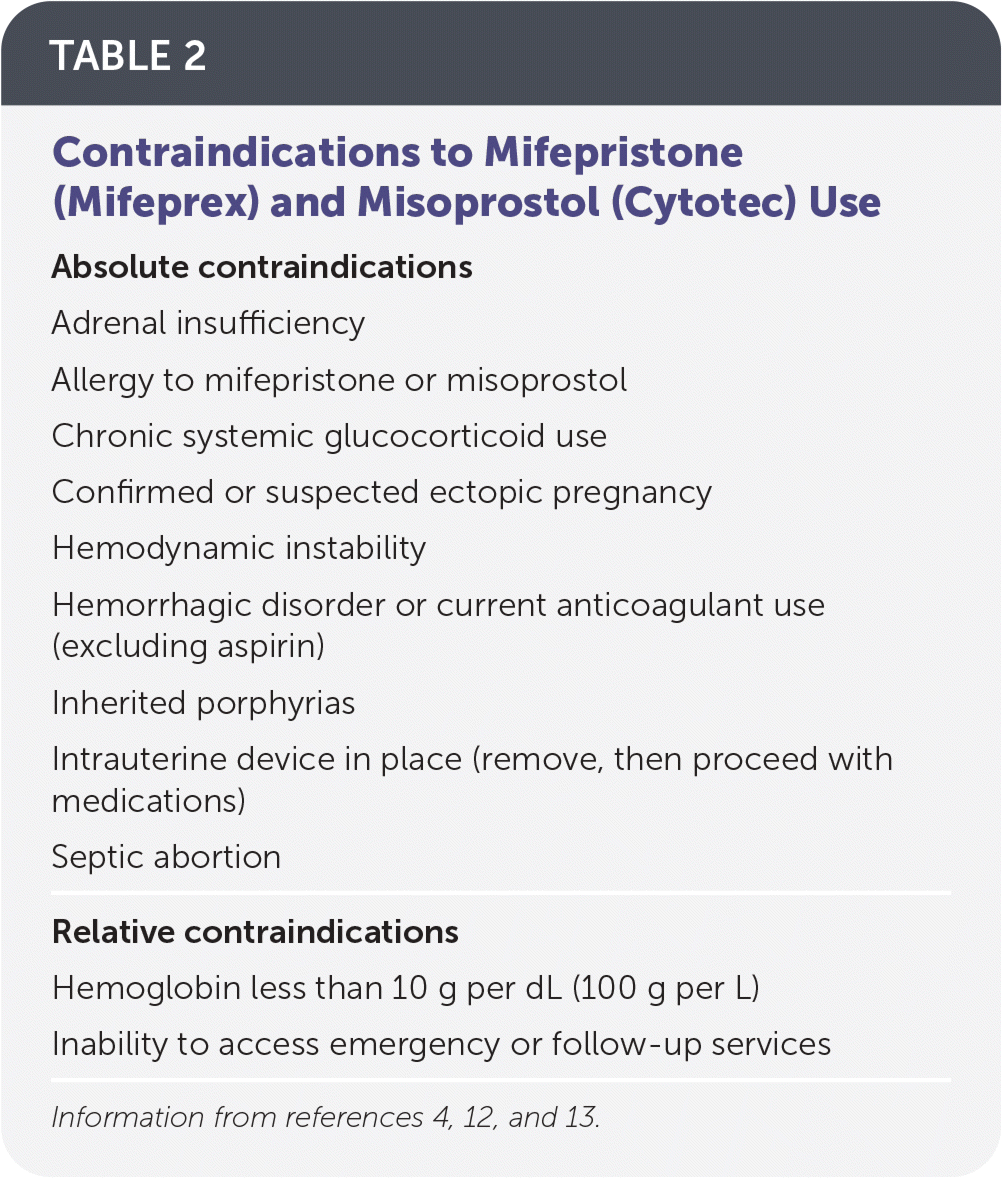
| Absolute contraindications |
| Adrenal insufficiency |
| Allergy to mifepristone or misoprostol |
| Chronic systemic glucocorticoid use |
| Confirmed or suspected ectopic pregnancy |
| Hemodynamic instability |
| Hemorrhagic disorder or current anticoagulant use (excluding aspirin) |
| Inherited porphyrias |
| Intrauterine device in place (remove, then proceed with medications) |
| Septic abortion |
| Relative contraindications |
| Hemoglobin less than 10 g per dL (100 g per L) |
| Inability to access emergency or follow-up services |
Providing Counseling and Consent
Patients with early pregnancy loss or unintended pregnancy should receive patient-centered counseling on all management options because patients who are included in the decision-making process and whose treatment preferences are honored have better mental health outcomes.17,18 The risks and benefits of treatment options for early pregnancy loss (i.e., expectant management, medication management, and uterine aspiration) are reviewed at https://www.aafp.org/afp/2019/0201/p166.html. For an in-depth discussion of the options for unintended pregnancy, including parenting, adoption, and medication or aspiration abortion, see https://www.aafp.org/afp/2015/0415/p544.html. All patients should be interviewed alone to ensure they are not being coerced by a partner or anyone else to decide against their will.19 The FDA requires patients who use mifepristone to sign a patient agreement that is available on the drug manufacturers' websites.20,21
Using Mifepristone and Misoprostol
REGIMENS FOR EARLY PREGNANCY LOSS
The most effective regimen for medication management of early pregnancy loss is 200 mg of oral mifepristone followed by 800 mcg of misoprostol administered vaginally 24 to 48 hours later.2,3 Regimens with misoprostol alone can be used if mifepristone is not available; however, rates of effectiveness are lower.2,3 One common regimen is misoprostol, 800 mcg vaginally, with a repeat dose in 48 hours if no bleeding has occurred22 (Table 32,5–8,21–28).
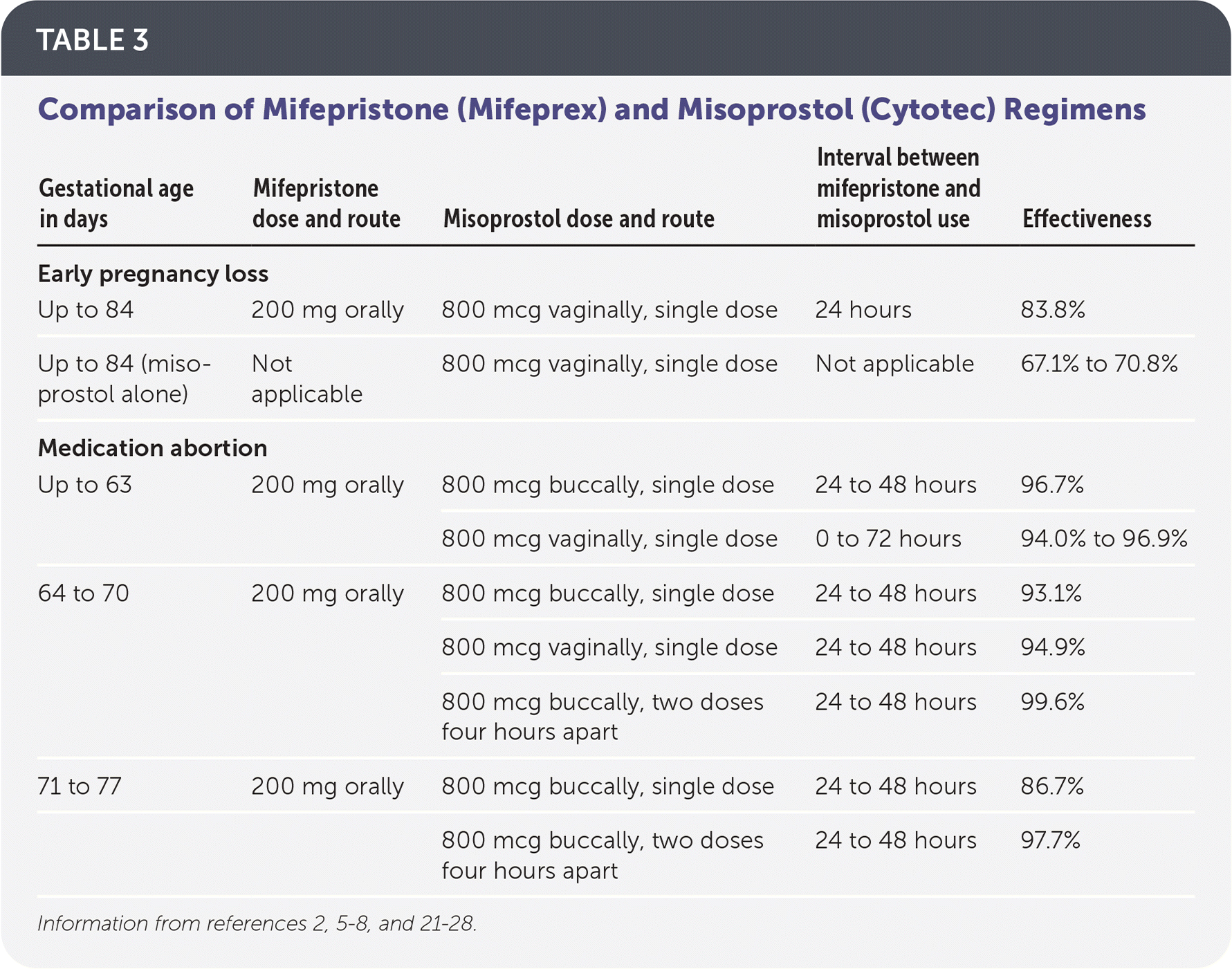
| Gestational age in days | Mifepristone dose and route | Misoprostol dose and route | Interval between mifepristone and misoprostol use | Effectiveness |
|---|---|---|---|---|
| Early pregnancy loss | ||||
| Up to 84 | 200 mg orally | 800 mcg vaginally, single dose | 24 hours | 83.8% |
| Up to 84 (misoprostol alone) | Not applicable | 800 mcg vaginally, single dose | Not applicable | 67.1% to 70.8% |
| Medication abortion | ||||
| Up to 63 | 200 mg orally | 800 mcg buccally, single dose | 24 to 48 hours | 96.7% |
| 800 mcg vaginally, single dose | 0 to 72 hours | 94.0% to 96.9% | ||
| 64 to 70 | 200 mg orally | 800 mcg buccally, single dose | 24 to 48 hours | 93.1% |
| 800 mcg vaginally, single dose | 24 to 48 hours | 94.9% | ||
| 800 mcg buccally, two doses four hours apart | 24 to 48 hours | 99.6% | ||
| 71 to 77 | 200 mg orally | 800 mcg buccally, single dose | 24 to 48 hours | 86.7% |
| 800 mcg buccally, two doses four hours apart | 24 to 48 hours | 97.7% | ||
REGIMENS FOR MEDICATION ABORTION
The FDA regimen for medication abortion up to 70 days' gestation is 200 mg of oral mifepristone followed by 800 mcg of misoprostol administered buccally 24 to 48 hours later.13,23 Evidence-based regimens, however, demonstrate safety and effectiveness up to 77 days' gestation.7,8,28,29 Effectiveness between 64 and 77 days' gestation increases with the addition of a second dose of misoprostol, 800 mcg four hours after the first dose.8,28,29 Other studies show that evidence-based regimens using vaginal misoprostol 0 to 72 hours after mifepristone administration are as safe, tolerable, and effective as the FDA regimen6,24–27 (Table 32,5–8,21–28).
PRESCRIBING LOGISTICS
Mifepristone must be ordered from the manufacturer and dispensed to the patient under the supervision of a clinician. Information on ordering mifepristone and resources for implementing medication management of early pregnancy loss or medication abortion are provided in Table 4. Telehealth has been shown to be a safe and effective model for providing medication abortion and may increase access.30 The patient may swallow the mifepristone in the office or at home. Home dosing allows for more flexible timing of subsequent misoprostol use and related cramping and bleeding.
| Resource | Website | Comments |
|---|---|---|
| National Abortion Federation 2020 Clinical Policy Guidelines for Abortion Care | https://prochoice.org/providers/quality-standards/ | Clinical guideline |
| Reproductive Health Access Project | https://www.reproductiveaccess.org/resource/order-mifepristone/ | Patient handouts and provider resources |
| https://www.reproductiveaccess.org/abortion/ | ||
| https://www.reproductiveaccess.org/resource/miscarriage-treatment-medication/ | ||
| https://www.reproductiveaccess.org/resource/mabfactsheet/ | ||
| Reproductive Health Education in Family Medicine | https://rhedi.org/education/medication-abortion/ | Curricular resources for medication abortion |
Misoprostol is available by prescription, or it can be stocked in the office. Patients using misoprostol buccally should place two tablets between the cheek and gums on each side of the mouth and allow them to dissolve for 30 minutes before swallowing any remaining medication. Patients using misoprostol vaginally should place four pills in the vagina and lie down for 30 minutes to allow the medication to be absorbed.
MANAGING EXPECTED AND ADVERSE EFFECTS
Mifepristone is generally well tolerated, with the most common adverse effect being nausea.23 Misoprostol causes strong uterine cramping and heavier bleeding than menses, often with blood clots. Cramping and bleeding typically begin within several hours of using misoprostol and last for three to five hours. Lighter bleeding persists for an average of nine to 16 days.4 Pain can usually be managed with nonsteroidal anti-inflammatory drugs and a heating pad.
Clinicians should inform patients that gastrointestinal symptoms such as nausea, vomiting, and diarrhea are common with misoprostol use. Oral antiemetics may be helpful. Low-grade fever and chills are less common and can be managed with antipyretics.23
Safety
Complications following treatment are rare and include hemorrhage, infection, ongoing pregnancy, and undiagnosed ectopic pregnancy (Table 5).2,22,23,31,32 For early pregnancy loss, the rate of unplanned aspiration attributed to persistent pain or bleeding is 8.8% when using combined regimens of mifepristone and misoprostol and 23.5% when using misoprostol alone.2 For patients undergoing medication abortion, rates of unanticipated uterine aspiration attributed to persistent pain or bleeding range from 1.8% to 4.2%.23 Prophylactic antibiotics are not recommended for medication management of early pregnancy loss or abortion.33
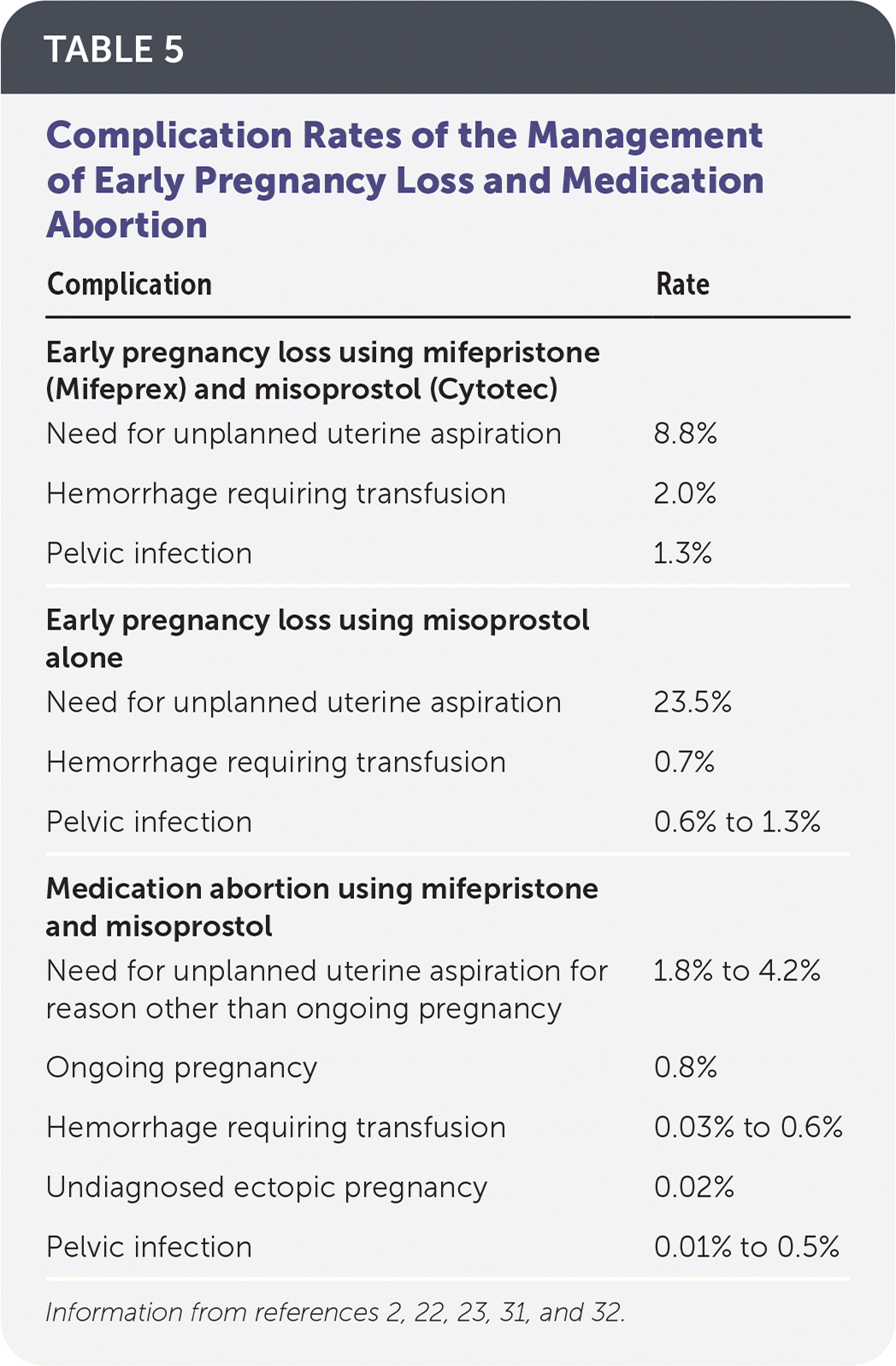
| Complication | Rate |
|---|---|
| Early pregnancy loss using mifepristone (Mifeprex) and misoprostol (Cytotec) | |
| Need for unplanned uterine aspiration | 8.8% |
| Hemorrhage requiring transfusion | 2.0% |
| Pelvic infection | 1.3% |
| Early pregnancy loss using misoprostol alone | |
| Need for unplanned uterine aspiration | 23.5% |
| Hemorrhage requiring transfusion | 0.7% |
| Pelvic infection | 0.6% to 1.3% |
| Medication abortion using mifepristone and misoprostol | |
| Need for unplanned uterine aspiration for reason other than ongoing pregnancy | 1.8% to 4.2% |
| Ongoing pregnancy | 0.8% |
| Hemorrhage requiring transfusion | 0.03% to 0.6% |
| Undiagnosed ectopic pregnancy | 0.02% |
| Pelvic infection | 0.01% to 0.5% |
Patients should be instructed to call if they experience symptoms of potential complications, including heavy bleeding, no bleeding following misoprostol use, pain not relieved by analgesics, purulent vaginal discharge, or fever or feeling ill more than 24 hours after using misoprostol. The differential diagnoses and triage for these symptoms are listed in Table 6.
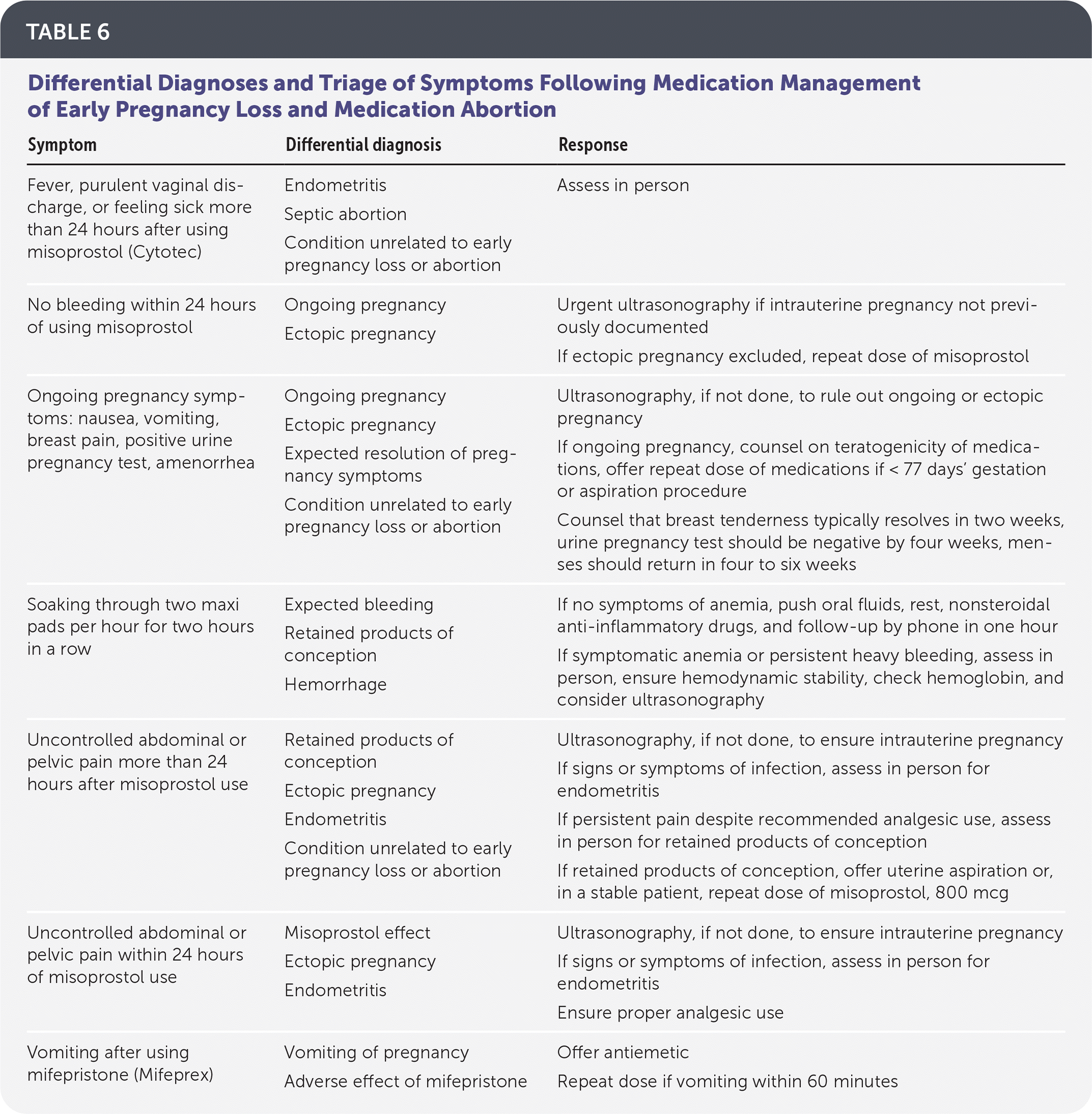
| Symptom | Differential diagnosis | Response |
|---|---|---|
| Fever, purulent vaginal discharge, or feeling sick more than 24 hours after using misoprostol (Cytotec) | Endometritis Septic abortion Condition unrelated to early pregnancy loss or abortion | Assess in person |
| No bleeding within 24 hours of using misoprostol | Ongoing pregnancy Ectopic pregnancy | Urgent ultrasonography if intrauterine pregnancy not previously documented If ectopic pregnancy excluded, repeat dose of misoprostol |
| Ongoing pregnancy symptoms: nausea, vomiting, breast pain, positive urine pregnancy test, amenorrhea | Ongoing pregnancy Ectopic pregnancy Expected resolution of pregnancy symptoms Condition unrelated to early pregnancy loss or abortion | Ultrasonography, if not done, to rule out ongoing or ectopic pregnancy If ongoing pregnancy, counsel on teratogenicity of medications, offer repeat dose of medications if < 77 days' gestation or aspiration procedure Counsel that breast tenderness typically resolves in two weeks, urine pregnancy test should be negative by four weeks, menses should return in four to six weeks |
| Soaking through two maxi pads per hour for two hours in a row | Expected bleeding Retained products of conception Hemorrhage | If no symptoms of anemia, push oral fluids, rest, nonsteroidal anti-inflammatory drugs, and follow-up by phone in one hour If symptomatic anemia or persistent heavy bleeding, assess in person, ensure hemodynamic stability, check hemoglobin, and consider ultrasonography |
| Uncontrolled abdominal or pelvic pain more than 24 hours after misoprostol use | Retained products of conception Ectopic pregnancy Endometritis Condition unrelated to early pregnancy loss or abortion | Ultrasonography, if not done, to ensure intrauterine pregnancy If signs or symptoms of infection, assess in person for endometritis If persistent pain despite recommended analgesic use, assess in person for retained products of conception If retained products of conception, offer uterine aspiration or, in a stable patient, repeat dose of misoprostol, 800 mcg |
| Uncontrolled abdominal or pelvic pain within 24 hours of misoprostol use | Misoprostol effect Ectopic pregnancy Endometritis | Ultrasonography, if not done, to ensure intrauterine pregnancy If signs or symptoms of infection, assess in person for endometritis Ensure proper analgesic use |
| Vomiting after using mifepristone (Mifeprex) | Vomiting of pregnancy Adverse effect of mifepristone | Offer antiemetic Repeat dose if vomiting within 60 minutes |
Based on a 2018 review, the National Academies of Sciences, Engineering, and Medicine concludes that medication abortion does not increase the risk of breast cancer, mental health problems, infertility, pregnancy loss, or preterm birth.4 Long-term fertility rates and pregnancy outcomes are similar for medication compared with surgical management of early pregnancy loss.34
Patient Follow-up
Successful passage of pregnancy tissue after early pregnancy loss or medication abortion should be confirmed by combining clinical history with a negative urine pregnancy test result, an adequate decline in serial serum β-hCG levels, or ultrasonography documenting the absence of a previously visible gestational sac.35 Serum β-hCG levels should fall by at least 50% in the first 24 hours or 80% by seven days after misoprostol use.11,35 Heterogeneous echogenicity, a thickened endometrial stripe, and the presence of Doppler flow on ultrasonography are not signs of incomplete abortion and, in the absence of symptoms, do not warrant further intervention.
Patients may start oral, transdermal, or vaginal contraception any time following misoprostol use. The etonogestrel implant (Nexplanon) can be inserted on the same day mifepristone is taken without increasing the risk of ongoing pregnancy.36 Medroxyprogesterone (Depo-Provera) and intrauterine devices may be used after confirmation of completed abortion.37 Patients who wish to conceive again can try as soon as they feel ready.38
Data Sources: A PubMed search was completed in Clinical Queries using the following key terms: medication abortion, early pregnancy loss, mifepristone, and misoprostol. The search included meta-analysis, randomized controlled trials, clinical trials, guidelines, and reviews. Also searched were the Cochrane database, the Agency for Healthcare Research and Quality, and DynaMed. An evidence summary, generated from Essential Evidence Plus, was reviewed, and relevant studies were referenced. Search dates: August 1 to November 1, 2019; and September 28, 2020.
The authors thank Allen Shaughnessy, PharmD, MMedEd, for his help in reviewing and editing the manuscript.
