
Am Fam Physician. 2013;87(8):556-566
Author disclosure: No relevant financial affiliations.
The American College of Chest Physicians provides recommendations for the use of anticoagulant medications for several indications that are important in the primary care setting. Warfarin, a vitamin K antagonist, is recommended for the treatment of venous thromboembolism and for the prevention of stroke in persons with atrial fibrillation, atrial flutter, or valvular heart disease. When warfarin therapy is initiated for venous thromboembolism, it should be given the first day, along with a heparin product or fondaparinux. The heparin product or fondaparinux should be continued for at least five days and until the patient's international normalized ratio is at least 2.0 for two consecutive days. The international normalized ratio goal and duration of treatment with warfarin vary depending on indication and risk. Warfarin therapy should be stopped five days before major surgery and restarted 12 to 24 hours postoperatively. Bridging with low-molecular-weight heparin or other agents is based on balancing the risk of thromboembolism with the risk of bleeding. Increasingly, self-testing is an option for selected patients on warfarin therapy. The ninth edition of the American College of Chest Physicians guidelines, published in 2012, includes a discussion of anticoagulants that have gained approval from the U.S. Food and Drug Administration since publication of the eighth edition in 2008. Dabigatran and apixaban are indicated for the prevention of systemic embolism and stroke in persons with nonvalvular atrial fibrillation. Rivaroxaban is indicated for the prevention of deep venous thrombosis in patients undergoing knee or hip replacement surgery, for treatment of deep venous thrombosis and pulmonary embolism, for reducing the risk of recurrent deep venous thrombosis and pulmonary embolism after initial treatment, and for prevention of systemic embolism in patients with nonvalvular atrial fibrillation.
Warfarin (Coumadin), unfractionated heparin, and low-molecular-weight heparin (LMWH) are commonly used for the prevention and treatment of disorders such as systemic embolism associated with atrial fibrillation, stroke, and venous thromboembolism (VTE). LMWH allows for the initiation of anticoagulation therapy on an outpatient basis.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Patients taking warfarin (Coumadin) should be treated using systematic processes of care to optimize effectiveness and minimize adverse effects. Health care professionals skilled in the initiation and assessment of therapy and dosing adjustments can dramatically influence outcomes. | B | 2, 3 |
| In patients with atrial fibrillation and at least one other risk factor for stroke, newer agents (rivaroxaban [Xarelto] and dabigatran [Pradaxa]) that do not require frequent laboratory monitoring are as effective as warfarin for prevention of stroke or systemic embolism and have comparable risks of major bleeding. | A | 11–19 |
| Compared with usual clinic-based care, patient self-testing for international normalized ratios, with or without self-dosing of warfarin, is associated with significantly fewer deaths and thromboembolic complications without any increase in bleeding complications for a selected group of motivated patients who have completed appropriate training. | A | 22–25 |
After decades during which warfarin was the only oral anticoagulation option, newer anticoagulants have the potential to change the management of coagulation disorders. This article focuses on the indications for and the goals and duration of anticoagulation therapy; describes methods to initiate therapy; and provides guidance on monitoring. Most of the recommendations are based on the American College of Chest Physicians (ACCP) evidence-based clinical practice guidelines (Table 1).1

| Medication | Recommendation | Implication for practice |
|---|---|---|
| Dabigatran (Pradaxa) |
|
|
| LMWH |
|
|
| Vitamin K |
|
|
| Warfarin |
|
|
|
| |
|
| |
|
|
Warfarin, Heparin, and Heparin Analogues
WARFARIN
The anticoagulant effect of warfarin results from the inhibition of the cyclic interconversion of vitamin K in the liver. The reduced form of vitamin K is necessary for the carboxylation of the terminal regions of the vitamin K proteins, factors II, VII, IX, and X.1 Without carboxylation, these vitamin K–dependent clotting factors do not become activated. Warfarin, similar in structure to vitamin K, interferes with the cyclic restoration of reduced levels of vitamin K. Therefore, warfarin indirectly reduces the synthesis of these clotting factors. The anticoagulant effects of warfarin are delayed for several days after dosing changes, including therapy initiation. This is because of the variable half-lives of previously formed circulating clotting factors. Carboxylation inhibition can also result in a paradoxical increased risk of clotting when warfarin is initiated because of decreased levels of the vitamin K–dependent anticoagulant proteins C and S.1
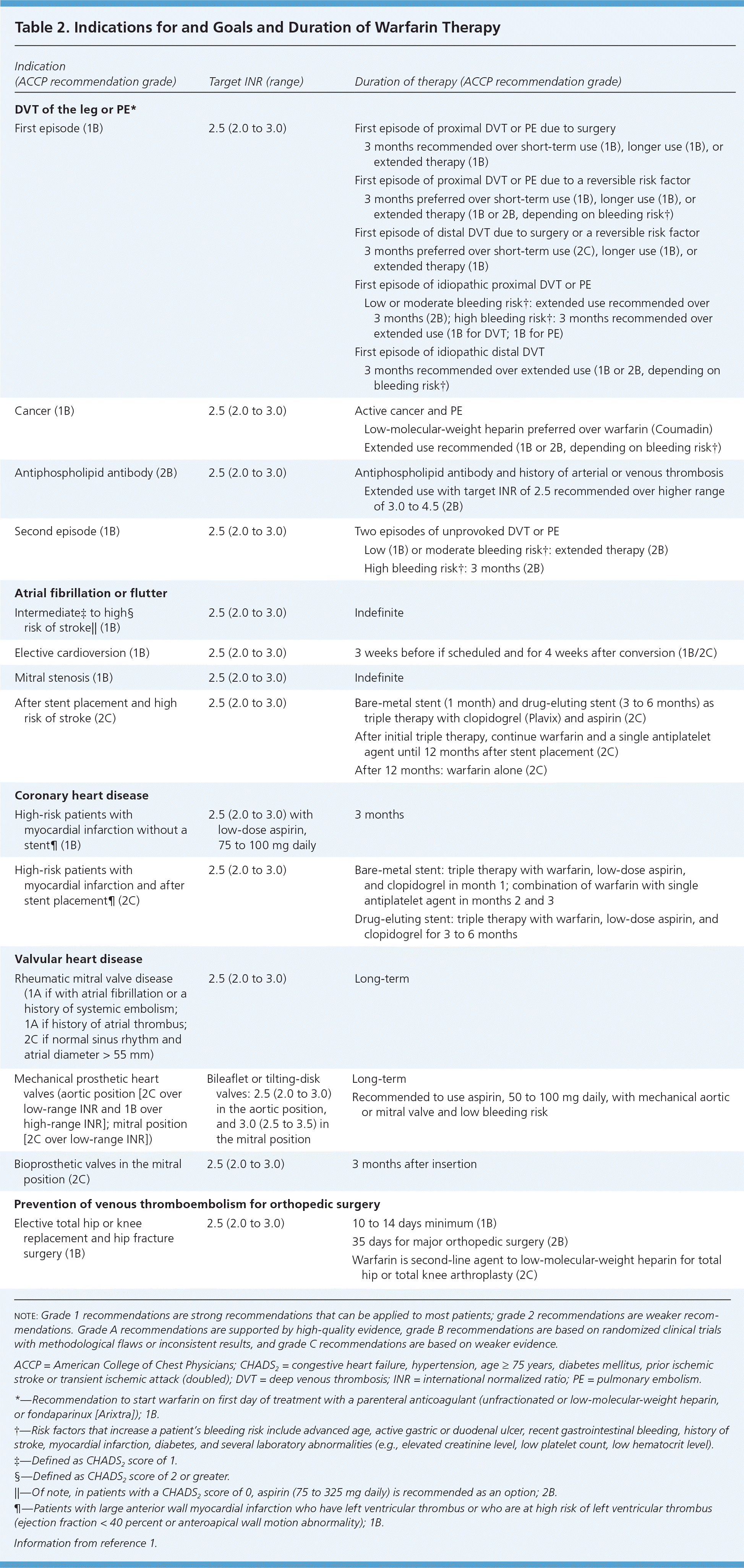
Indications. Indications for initiating warfarin are listed in Table 2.1 In persons with nonvalvular atrial fibrillation, clinicians often base the decision to start warfarin or LMWH on clinical risk estimates, such as the CHADS2 score, which assigns one point each for congestive heart failure, hypertension, age 75 years and older, and diabetes mellitus, and two points for prior ischemic stroke or transient ischemic attack.1 For persons with a CHADS2 score of 2 or higher, the ACCP guidelines recommend oral anticoagulation, and for persons with a score of 1, the guidelines recommend individualization of therapy and suggest oral anticoagulation rather than a combination of aspirin and clopidogrel (Plavix).1
| Indication (ACCP recommendation grade) | Target INR (range) | Duration of therapy (ACCP recommendation grade) | |
|---|---|---|---|
| DVT of the leg or PE* | |||
| First episode (1B) | 2.5 (2.0 to 3.0) | First episode of proximal DVT or PE due to surgery | |
| 3 months recommended over short-term use (1B), longer use (1B), or extended therapy (1B) | |||
| First episode of proximal DVT or PE due to a reversible risk factor | |||
| 3 months preferred over short-term use (1B), longer use (1B), or extended therapy (1B or 2B, depending on bleeding risk†) | |||
| First episode of distal DVT due to surgery or a reversible risk factor | |||
| 3 months preferred over short-term use (2C), longer use (1B), or extended therapy (1B) | |||
| First episode of idiopathic proximal DVT or PE | |||
| Low or moderate bleeding risk†: extended use recommended over3 months (2B); high bleeding risk†: 3 months recommended over extended use (1B for DVT; 1B for PE) | |||
| First episode of idiopathic distal DVT | |||
| 3 months recommended over extended use (1B or 2B, depending on bleeding risk†) | |||
| Cancer (1B) | 2.5 (2.0 to 3.0) | Active cancer and PE | |
| Low-molecular-weight heparin preferred over warfarin (Coumadin) | |||
| Extended use recommended (1B or 2B, depending on bleeding risk†) | |||
| Antiphospholipid antibody (2B) | 2.5 (2.0 to 3.0) | Antiphospholipid antibody and history of arterial or venous thrombosis | |
| Extended use with target INR of 2.5 recommended over higher range of 3.0 to 4.5 (2B) | |||
| Second episode (1B) | 2.5 (2.0 to 3.0) | Two episodes of unprovoked DVT or PE | |
| Low (1B) or moderate bleeding risk†: extended therapy (2B) | |||
| High bleeding risk†: 3 months (2B) | |||
| Atrial fibrillation or flutter | |||
| Intermediate‡ to high§ risk of stroke|| (1B) | 2.5 (2.0 to 3.0) | Indefinite | |
| Elective cardioversion (1B) | 2.5 (2.0 to 3.0) | 3 weeks before if scheduled and for 4 weeks after conversion (1B/2C) | |
| Mitral stenosis (1B) | 2.5 (2.0 to 3.0) | Indefinite | |
| After stent placement and high risk of stroke (2C) | 2.5 (2.0 to 3.0) | Bare-metal stent (1 month) and drug-eluting stent (3 to 6 months) as triple therapy with clopidogrel (Plavix) and aspirin (2C) | |
| After initial triple therapy, continue warfarin and a single antiplatelet agent until 12 months after stent placement (2C) | |||
| After 12 months: warfarin alone (2C) | |||
| Coronary heart disease | |||
| High-risk patients with myocardial infarction without a stent¶ (1B) | 2.5 (2.0 to 3.0) with low-dose aspirin, 75 to 100 mg daily | 3 months | |
| High-risk patients with myocardial infarction and after stent placement¶ (2C) | 2.5 (2.0 to 3.0) | Bare-metal stent: triple therapy with warfarin, low-dose aspirin, and clopidogrel in month 1; combination of warfarin with single antiplatelet agent in months 2 and 3 | |
| Drug-eluting stent: triple therapy with warfarin, low-dose aspirin, and clopidogrel for 3 to 6 months | |||
| Valvular heart disease | |||
| Rheumatic mitral valve disease (1A if with atrial fibrillation or a history of systemic embolism; 1A if history of atrial thrombus; 2C if normal sinus rhythm and atrial diameter > 55 mm) | 2.5 (2.0 to 3.0) | Long-term | |
| Mechanical prosthetic heart valves (aortic position [2C over low-range INR and 1B over high-range INR]; mitral position [2C over low-range INR]) | Bileaflet or tilting-disk valves: 2.5 (2.0 to 3.0) in the aortic position, and 3.0 (2.5 to 3.5) in the mitral position | Long-term | |
| Recommended to use aspirin, 50 to 100 mg daily, with mechanical aortic or mitral valve and low bleeding risk | |||
| Bioprosthetic valves in the mitral position (2C) | 2.5 (2.0 to 3.0) | 3 months after insertion | |
| Prevention of venous thromboembolism for orthopedic surgery | |||
| Elective total hip or knee replacement and hip fracture surgery (1B) | 2.5 (2.0 to 3.0) | 10 to 14 days minimum (1B) | |
| 35 days for major orthopedic surgery (2B) | |||
| Warfarin is second-line agent to low-molecular-weight heparin for total hip or total knee arthroplasty (2C) | |||
When warfarin is initiated, the international normalized ratio (INR) may begin to respond after two to three days because of the depletion of factor VII. During this initial period, the patient can enter a hypercoagulable state caused by warfarin's effects on proteins C and S.1 Heparin or LMWH should be administered with warfarin initiation and continued until the INR has been in the targeted therapeutic range for a minimum of 24 hours. Initial dosing of warfarin can vary depending on individual patient factors (e.g., age, bleeding risk, medication compliance history) and anticipated drug interactions.
In most patients, warfarin should be initiated as a maintenance dosage of 5 mg daily. Older patients and persons with liver disease, poor nutritional status, or heart failure may require lower initiation dosages.1 For persons who are healthy enough to be treated as outpatients, the ACCP guidelines provide an alternative warfarin initiation dosage of 10 mg daily for the first two days of therapy, rather than the anticipated maintenance dosage.1
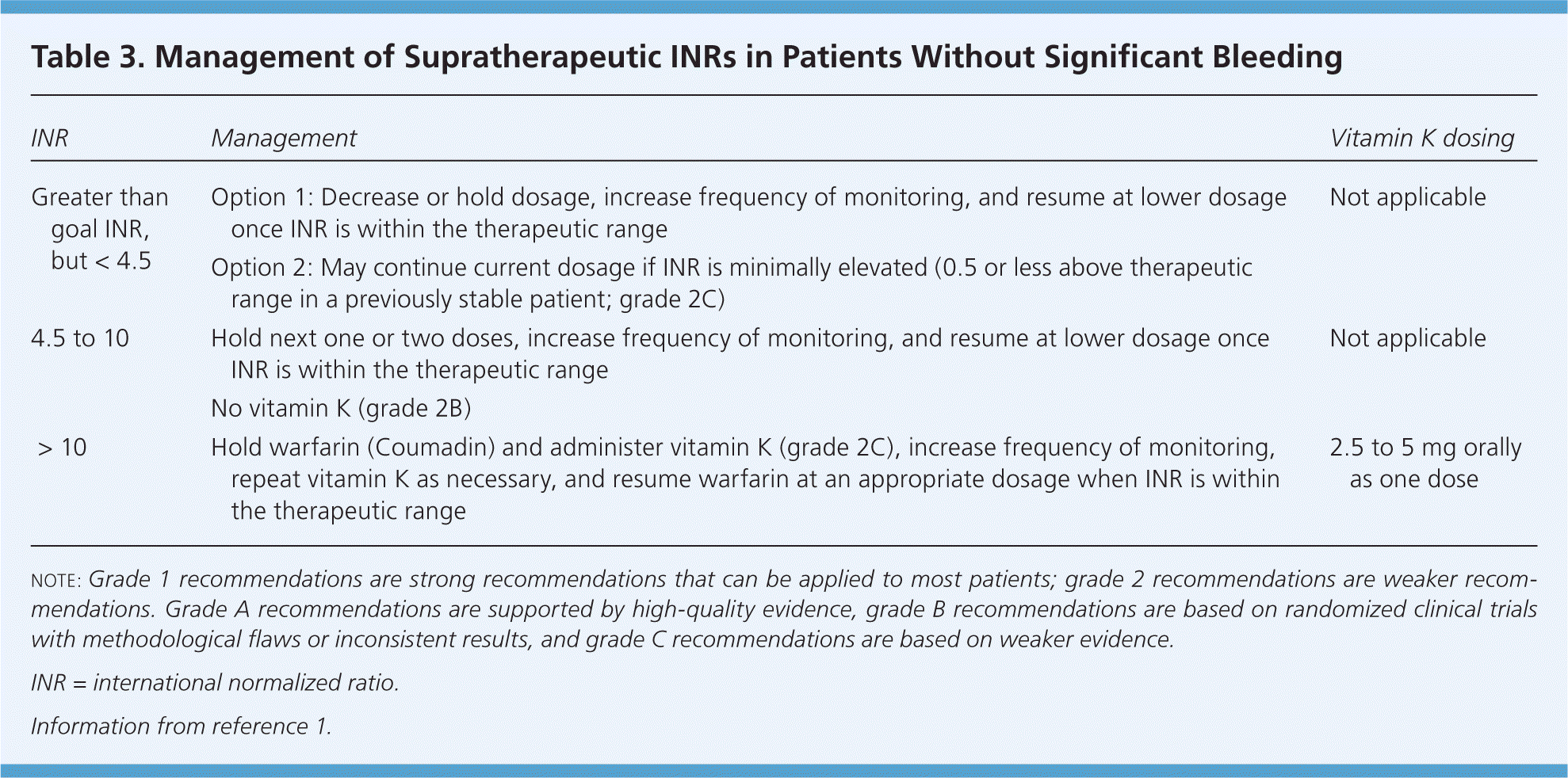
After baseline INR is determined, the next INR can be obtained after the patient has received two or three doses. Then, the frequency of INR monitoring decreases to twice weekly until the INR is within the therapeutic range, then weekly, every other week, and finally monthly.1 The ACCP guidelines allow clinicians to consider INR monitoring up to every 12 weeks in patients who are stable (defined as having at least three months of consistent results with no need to adjust warfarin dosing). If a patient's INR becomes subtherapeutic or supratherapeutic, the frequency of monitoring should be increased until it stabilizes again. The ACCP provides recommendations for managing supratherapeutic INRs (Table 3).1
| INR | Management | Vitamin K dosing |
|---|---|---|
| Greater than goal INR, but < 4.5 | Option 1: Decrease or hold dosage, increase frequency of monitoring, and resume at lower dosage once INR is within the therapeutic range | Not applicable |
| Option 2: May continue current dosage if INR is minimally elevated (0.5 or less above therapeutic range in a previously stable patient; grade 2C) | ||
| 4.5 to 10 | Hold next one or two doses, increase frequency of monitoring, and resume at lower dosage once INR is within the therapeutic range | Not applicable |
| No vitamin K (grade 2B) | ||
| > 10 | Hold warfarin (Coumadin) and administer vitamin K (grade 2C), increase frequency of monitoring, repeat vitamin K as necessary, and resume warfarin at an appropriate dosage when INR is within the therapeutic range | 2.5 to 5 mg orally as one dose |
Patients taking warfarin in the evening can adjust their dosing based on that day's INR results. If the INR is not within the desired therapeutic range after excluding explanatory factors, a 5 to 20 percent increase or decrease in the total weekly dosage is required.4–6 Patients should be provided with the simplest regimen to achieve the new total weekly dosage.
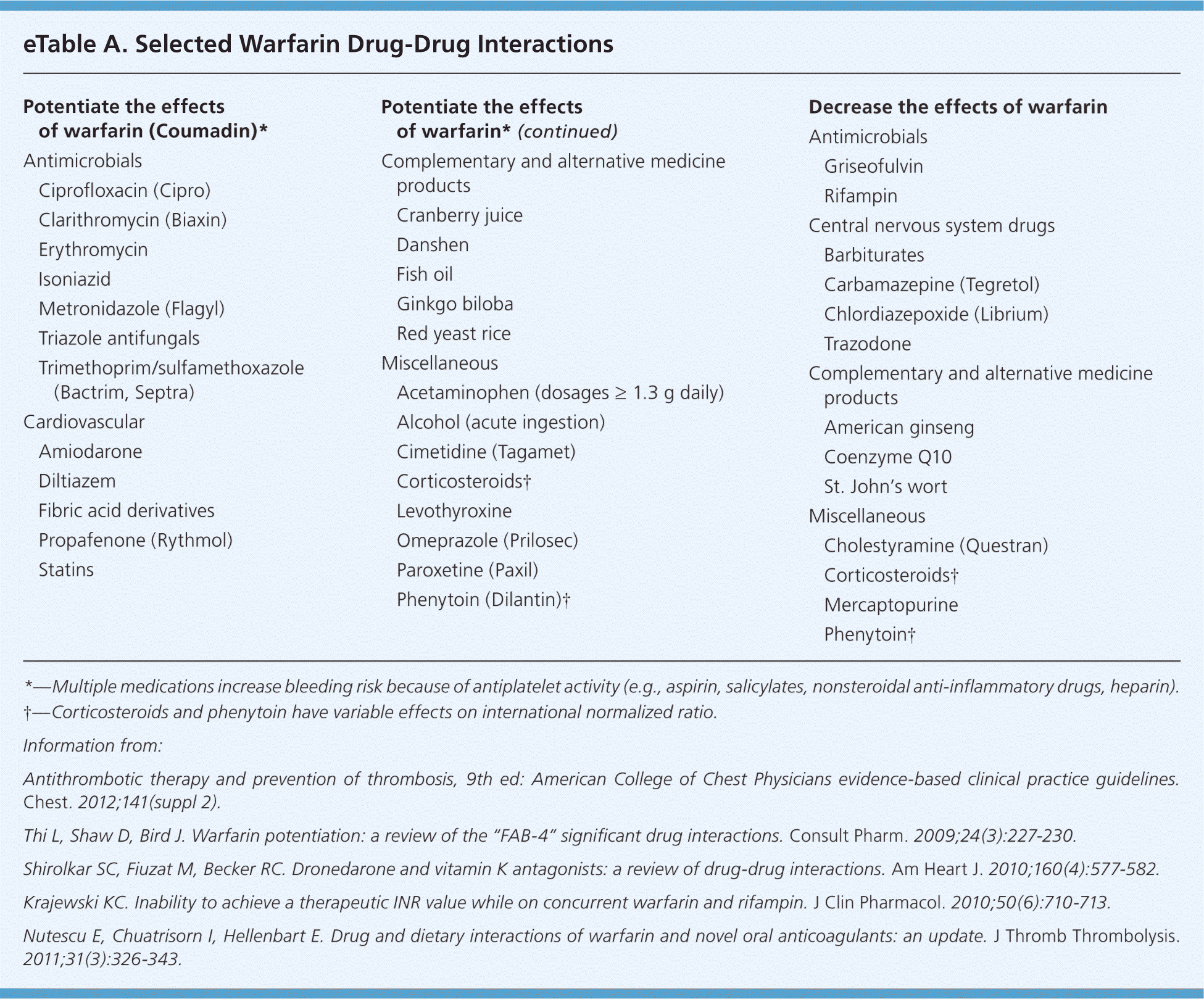
Drug, Food, and Disease State Interactions. Warfarin is subject to many drug-drug, drug-food, and drug–disease state interactions. eTable A lists selected drug-drug interactions that are considered highly likely to potentiate or inhibit the effects of warfarin.
Potentiate the effects of warfarin (Coumadin)*
|
Some medications, such as amiodarone and rifampin, can impact a patient's INR long after the medication is discontinued.1,7,8 A patient taking a medication with higher interaction potential, such as metronidazole (Flagyl), should be monitored more frequently.9 Depending on the patient and the medication, a prophylactic reduction in warfarin dosage may also be advised.
Medical conditions such as diarrhea, heart failure, fever, hyperthyroidism, and liver disease can potentiate warfarin's effects. Conversely, conditions such as hypothyroidism can decrease the expected effects of warfa-rin.1 Genetic factors can predispose patients to reduced warfarin requirements, as well as warfarin resistance. Although there is a small subset of patients who may have unexpected responses to warfarin, it is not currently recommended that patients undergo genetic testing.1
UNFRACTIONATED HEPARIN
| Dosage | Dosage adjustment in patientswith renal impairment | Half-life | Reversibility | Monitoring | Comparisons |
|---|---|---|---|---|---|
| Unfractionated heparin*† | |||||
|
| 0.5 to2 hours | Protamine |
| Unfractionated heparin vs. LMWH
|
| LMWH* | |||||
Enoxaparin (Lovenox)
|
| 3 to 6 hours | NA |
| Unfractionated heparin vs. LMWH
|
Dalteparin (Fragmin)†
|
| 3 to 5 hours | NA | ||
Tinzaparin (Innohep)
|
| 3 to 4 hours | NA | ||
| Fondaparinux (Arixtra) | |||||
|
| 18 hours | NA |
| LMWH vs. fondaparinux
|
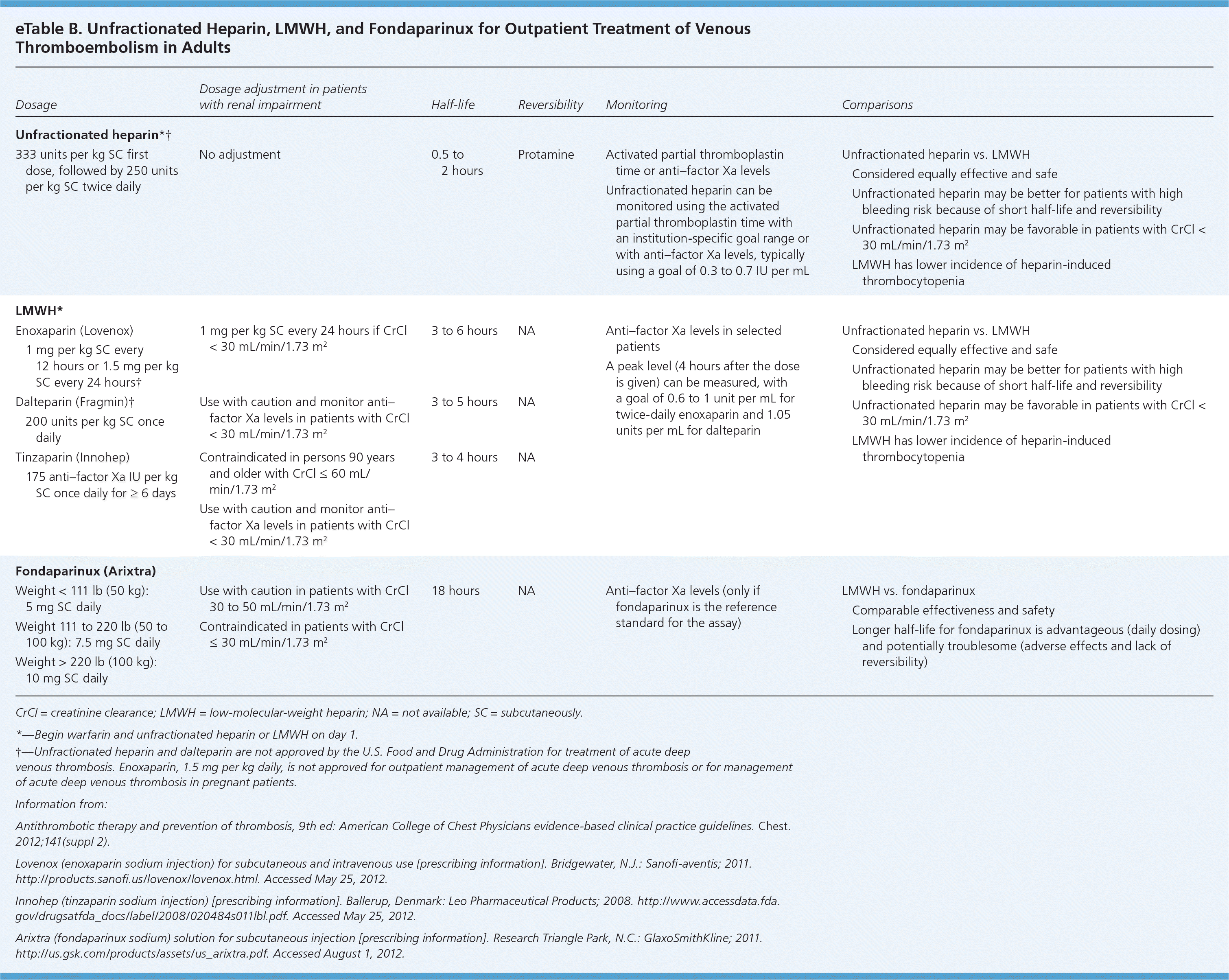
Beyond increased bleeding risk, unfractionated heparin is associated with other adverse effects, such as heparin-induced thrombocytopenia. Heparin-induced thrombocytopenia should be suspected if a patient's platelet count decreases by at least 50 percent, or is less than 150 × 103 per μL after initiation of heparin. This generally occurs five to 14 days after initiation, and can occur after heparin is discontinued.
LOW-MOLECULAR-WEIGHT HEPARIN
Two LMWHs, dalteparin (Fragmin) and enoxaparin (Lovenox), are commonly used in clinical practice. LMWH is derived from unfractionated heparin and has an increased affinity for factor Xa relative to thrombin.1 LMWH, which is given subcutaneously, has predictable absorption and degree of anticoagulation. Monitoring with measurement of anti–factor Xa levels is not routinely recommended, but is potentially useful in certain situations in which predictability of the degree of anticoagulation may be altered, such as changes in pharmacokinetics and pharmacodynamics (e.g., obesity, pregnancy) and accumulation (e.g., older age, kidney disease). In the outpatient setting, the usefulness of laboratory testing is limited to the assessment of bleeding events and therapeutic failures. It may also be of value to assess levels infrequently during the course of long-term therapy (i.e., when LMWH is used for more than just bridging therapy). Although LMWH has a similar bleeding risk and lower heparin-induced thrombocytopenia risk compared with unfractionated heparin, a patient with a history of heparin-induced thrombocytopenia should not take LMWH.1
FONDAPARINUX
Fondaparinux (Arixtra) is a synthetic analogue of heparin. Unlike LMWH, fondaparinux is specific only to factor Xa and has no effect on thrombin formation. Like LMWH, fondaparinux is given subcutaneously and has predictable absorption and degree of anticoagulation. The ACCP guidelines recommend fondaparinux for general surgical prophylaxis in patients who have contraindications to LMWH.1
There are few data on the monitoring of fondaparinux. Anti–factor Xa levels can be used as long as fondaparinux (and not LMWH) is the reference standard in the assay.1 Although the bleeding risk is similar to unfractionated heparin and LMWH, there is little heparin-induced thrombocytopenia risk with fondaparinux.
Bridging Unfractionated Heparin, LMWH, or Fondaparinux to Warfarin
In the treatment of VTE and pulmonary embolism, the parenteral anticoagulant should be overlapped with warfarin for a minimum of five days. In most cases, warfarin can be initiated on day 1, after the first dose of the parenteral agent has been given. Warfarin should not be initiated alone, and the parenteral anticoagulant should not be discontinued until the INR is in the therapeutic range for two consecutive days.
Depending on the patient's risk of thromboembolism and bleeding, bridging should occur when a patient's oral anticoagulation therapy needs to be interrupted (eTable C). Interruption is common in patients undergoing surgery. For most persons who are not having a minor procedure, warfarin will be stopped approximately five days before surgery and restarted 12 to 24 hours postoperatively. LMWH should be restarted approximately 24 hours after the procedure, and it may be prudent to wait 48 to 72 hours before resuming the medication for patients at high risk of bleeding or who are undergoing major surgery.1 Fondaparinux is not recommended for this indication.
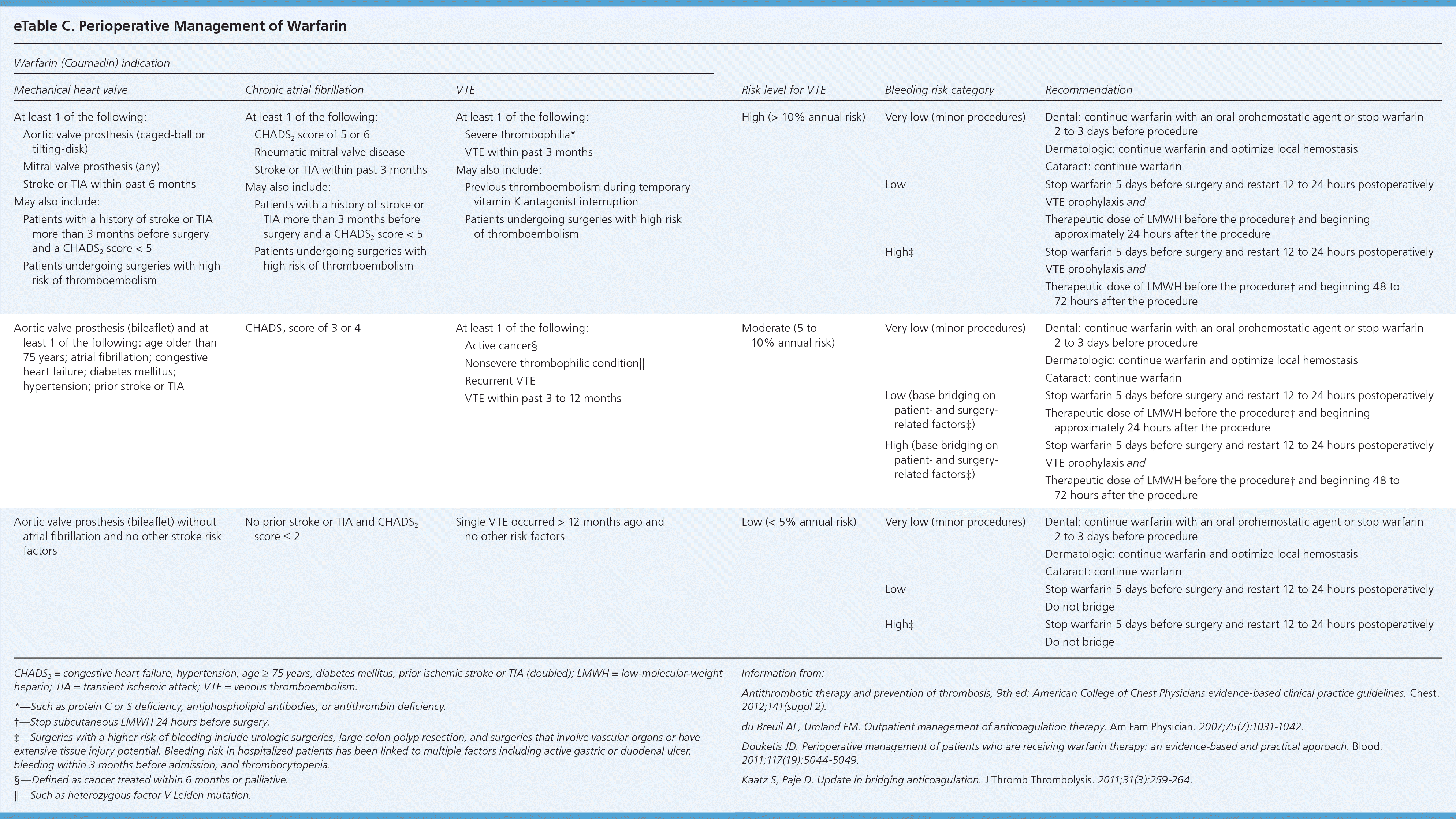
| Warfarin (Coumadin) indication | |||||
|---|---|---|---|---|---|
| Mechanical heart valve | Chronic atrial fibrillation | VTE | Risk level for VTE | Bleeding risk category | Recommendation |
At least 1 of the following:
| At least 1 of the following:
| At least 1 of the following:
| High (> 10% annual risk) | Very low (minor procedures) | Dental: continue warfarin with an oral prohemostatic agent or stop warfarin2 to 3 days before procedure |
| Dermatologic: continue warfarin and optimize local hemostasis | |||||
| Cataract: continue warfarin | |||||
| Low | Stop warfarin 5 days before surgery and restart 12 to 24 hours postoperatively | ||||
| VTE prophylaxis and | |||||
| Therapeutic dose of LMWH before the procedure† and beginningapproximately 24 hours after the procedure | |||||
| High‡ | Stop warfarin 5 days before surgery and restart 12 to 24 hours postoperatively | ||||
| VTE prophylaxis and | |||||
| Therapeutic dose of LMWH before the procedure† and beginning 48 to72 hours after the procedure | |||||
| Aortic valve prosthesis (bileaflet) and atleast 1 of the following: age older than75 years; atrial fibrillation; congestiveheart failure; diabetes mellitus;hypertension; prior stroke or TIA | CHADS2 score of 3 or 4 | At least 1 of the following:
| Moderate (5 to10% annual risk) | Very low (minor procedures) | Dental: continue warfarin with an oral prohemostatic agent or stop warfarin2 to 3 days before procedure |
| Dermatologic: continue warfarin and optimize local hemostasis | |||||
| Cataract: continue warfarin | |||||
| Low (base bridging onpatient- and surgeryrelatedfactors‡) | Stop warfarin 5 days before surgery and restart 12 to 24 hours postoperatively | ||||
| Therapeutic dose of LMWH before the procedure† and beginningapproximately 24 hours after the procedure | |||||
| High (base bridging onpatient- and surgeryrelatedfactors‡) | Stop warfarin 5 days before surgery and restart 12 to 24 hours postoperatively | ||||
| VTE prophylaxis and | |||||
| Therapeutic dose of LMWH before the procedure† and beginning 48 to72 hours after the procedure | |||||
| Aortic valve prosthesis (bileaflet) withoutatrial fibrillation and no other stroke riskfactors | No prior stroke or TIA and CHADS2score ≤ 2 | Single VTE occurred > 12 months ago andno other risk factors | Low (< 5% annual risk) | Very low (minor procedures) | Dental: continue warfarin with an oral prohemostatic agent or stop warfarin2 to 3 days before procedure |
| Dermatologic: continue warfarin and optimize local hemostasis | |||||
| Cataract: continue warfarin | |||||
| Low | Stop warfarin 5 days before surgery and restart 12 to 24 hours postoperatively | ||||
| Do not bridge | |||||
| High‡ | Stop warfarin 5 days before surgery and restart 12 to 24 hours postoperatively | ||||
| Do not bridge | |||||
For all warfarin indications, perioperative bridging is not indicated in patients at low risk of thromboembolism.1 For patients with a high risk of thromboembolism, bridging with a therapeutic dose of unfractionated heparin or LMWH is indicated.1 The ACCP guidelines are less clear about how patients with a moderate risk of thromboembolism should be treated.1 Clinicians need to balance the individual's risk of thromboembolism, based on the medical history and surgical procedure, and risk of bleeding when determining what is optimal in persons in this moderate-risk category.
Newer Anticoagulants
The effectiveness of warfarin is well-established; however, it is a suboptimal anticoagulant because it requires frequent monitoring and dosage adjustments, and because of its potential for multiple drug-drug, drug-food, and drug–disease state interactions. It has a lengthy half-life and a delayed anticoagulant effect, and it often requires bridging therapy.
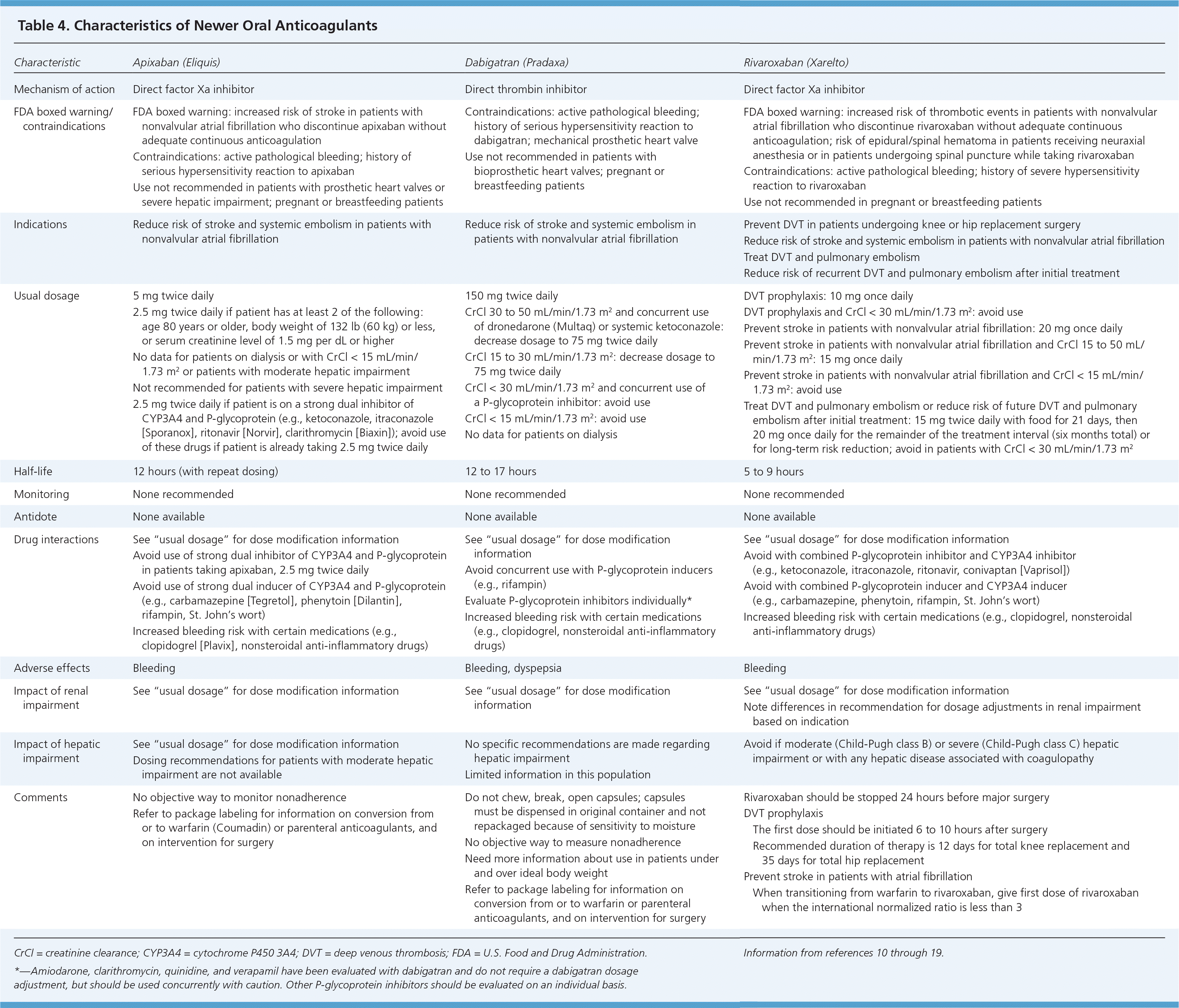
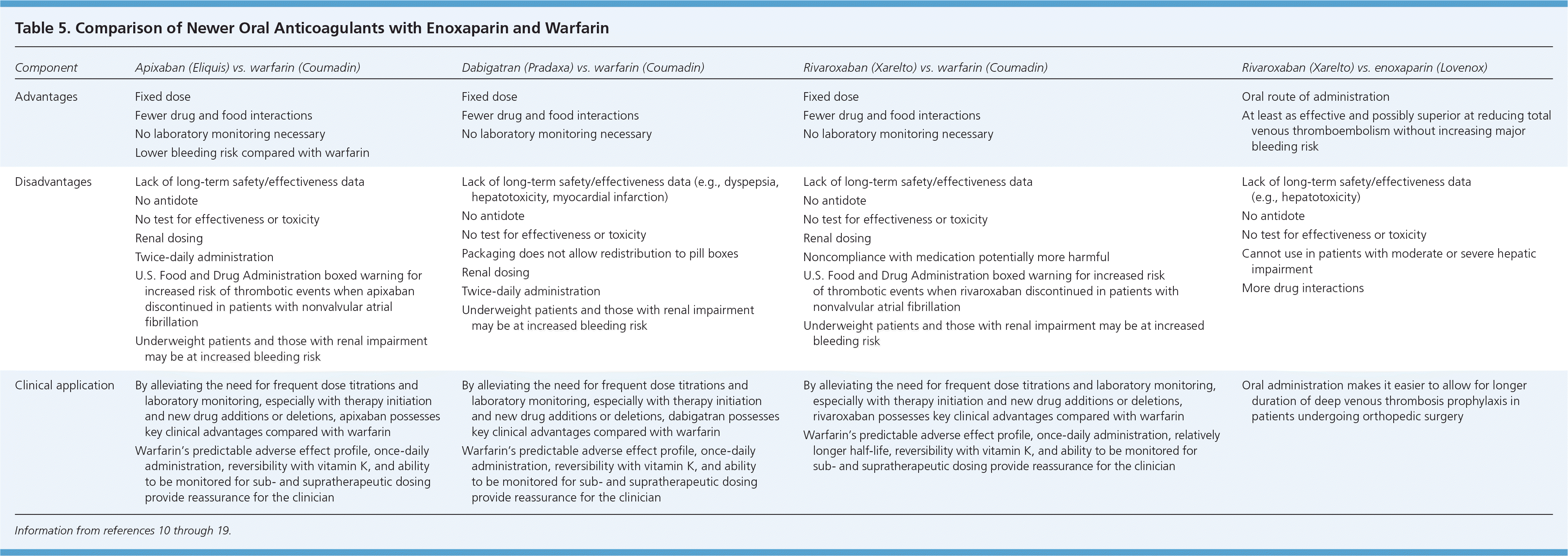
Since the approval of warfarin in 1954, no other oral option existed for patients who needed long-term anticoagulation therapy. This changed in 2010 with the U.S. Food and Drug Administration (FDA) approval of the oral direct thrombin inhibitor dabigatran (Pradaxa), in 2011 with the FDA approval of the oral direct factor Xa inhibitor rivaroxaban (Xarelto), and again in 2012 with the FDA approval of the oral factor Xa inhibitor apixaban (Eliquis). Characteristics of these anticoagulants are provided in Tables 4 and 5.10–19
| Characteristic | Apixaban (Eliquis) | Dabigatran (Pradaxa) | Rivaroxaban (Xarelto) | |
|---|---|---|---|---|
| Mechanism of action |
|
|
| |
| FDA boxed warning/contraindications |
|
|
| |
| Indications |
|
|
| |
| Usual dosage |
|
|
| |
| Half-life |
|
|
| |
| Monitoring |
|
|
| |
| Antidote |
|
|
| |
| Drug interactions |
|
|
| |
| Adverse effects |
|
|
| |
| Impact of renal impairment |
|
|
| |
| Impact of hepatic impairment |
|
|
| |
| Comments |
|
|
| |
| Component | Apixaban (Eliquis) vs. warfarin (Coumadin) | Dabigatran (Pradaxa) vs. warfarin (Coumadin) | Rivaroxaban (Xarelto) vs. warfarin (Coumadin) | Rivaroxaban (Xarelto) vs. enoxaparin (Lovenox) |
|---|---|---|---|---|
| Advantages |
|
|
|
|
| Disadvantages |
|
|
|
|
| Clinical application |
|
|
|
|
DABIGATRAN
Dabigatran is available as a fixed-dose medication for the prevention of systemic embolism and stroke in patients with nonvalvular atrial fibrillation.13 The ACCP guidelines recommend dabigatran, 150 mg twice daily, over warfarin for this indication when dosed appropriately for the patient's renal function.1 Dabigatran does not require monitoring, dosage adjustments, or overlap with injectable anticoagulants such as heparin. Without applicable laboratory monitoring, there is no mechanism to establish if a patient's INR is subtherapeutic or supratherapeutic. If the patient's INR is supratherapeutic, there is no antidote for reversal. This can be problematic when determining the appropriate management in a patient who needs emergent surgery. The short half-life is potentially challenging when assessing the impact of noncompliance or missing the second daily dose. Limited data are available for patients with hepatic impairment and for patients who are obese. Thus, it is not acceptable to automatically consider all patients taking warfarin to be good candidates for dabigatran.
Adverse effects of dabigatran, 150 mg twice daily, compared with warfarin include dyspepsia (11.3 versus 5.8 percent; P < .001) and major gastrointestinal bleeding (1.51 versus 1.02 percent; P < .001). Dabigatran's safety profile needs further evaluation.13
Elevated transaminase levels in the Randomized Evaluation of Long-term Anticoagulation Therapy trial were comparable to those seen with warfarin, and routine liver function test monitoring is not recommended.11,13 Because of the effects of renal function on dabigatran, baseline and periodic renal function monitoring are recommended.
RIVAROXABAN
Rivaroxaban is indicated for prevention of deep venous thrombosis in patients undergoing knee or hip replacement surgery, for treatment of deep venous thrombosis and pulmonary embolism, for reducing the risk of recurrent deep venous thrombosis and pulmonary embolism after initial treatment, and for prevention of systemic embolism in patients with nonvalvular atrial fibrillation. It is expected to prolong the activated partial thromboplastin time and increase anti–factor Xa levels; however, the usefulness of monitoring has not been established. In four trials evaluating the role of rivaroxaban in the prevention of VTE in patients undergoing orthopedic surgery, rivaroxaban significantly reduced the primary outcome (total VTE and all-cause mortality) compared with enoxaparin, without significantly increasing bleeding risk.14–19 In the ROCKET AF trial, rivaroxaban was shown to be noninferior to warfarin for the prevention of stroke or systemic embolism in patients with nonvalvular atrial fibrillation.19 An important consideration in this trial is that in the warfarin arm, the time in therapeutic range was 55 percent, which is less than what is typically reported by anticoagulation clinics.
Rivaroxaban has been studied for the treatment of acute coronary syndromes. Similar to dabigatran, baseline and periodic renal function monitoring are recommended.
APIXABAN
Similar to dabigatran, apixaban is also indicated for the prevention of systemic embolism and stroke in patients with nonvalvular atrial fibrillation. In the ARISTOTLE and AVERROES trials, apixaban reduced the primary outcome of ischemic stroke, hemorrhagic stroke, and systemic embolism compared with warfarin and aspirin, respectively. There were also lower mortality rates in the apixaban group in both trials and a lower major bleeding rate in the apixaban group compared with warfarin in the ARISTOTLE trial.20,21
Patient Self-Testing
Point-of-care monitors are typically used in primary care and anticoagulation clinics and have several advantages, including rapid INR acquisition and interpretation. These monitors make it possible for patients to check their INRs at home, which is referred to as patient self-testing. Reassuring data exist for the effective use of patient self-testing in selected patients who demonstrate monitor competency. Decreased mortality, enhanced INR control, decreased thromboembolic events, and an improvement in patient satisfaction and quality of life have been demonstrated with patient self-testing, all without an increase in bleeding complications.22–24 In 2008, the Centers for Medicare and Medicaid Services expanded its patient self-testing coverage,25 which is outlined in eTable D. The cost of patient self-testing, which is similar to the cost of newer oral anticoagulants, can be significant without reimbursement; however, self-testing is not appropriate for all patients on warfarin therapy22–24 (eTable D).

| Centers for Medicare and Medicaid Services coverage includes patients: |
| In whom self-testing is prescribed by a physician |
| Taking warfarin (Coumadin) for long-term anticoagulation for venousthromboembolism, mechanical heart valves, or atrial fibrillation |
| Taking warfarin for at least three months before initiation of self-testing |
| Who do not require testing frequency greater than once weekly |
| Who participate in, and successfully complete, an anticoagulation educationprogram* |
| Patients who may not be good self-testing candidates include those: |
| Who do not demonstrate monitor or instruction competency† |
| Who will be treated with warfarin for fewer than six months |
| With atypical international normalized ratio target ranges |
| With intellectual impairment |
| With known drug or alcohol abuse |
| With language barriers that cannot be overcome with the assistance of a familymember |
Data Sources: A PubMed search was completed in Clinical Queries using the key terms outpatient, anticoagulation, warfarin, dabigatran, rivaroxaban, heparin, low-molecular-weight heparin, dalteparin, enoxaparin, patient self-monitor, and INR. The search included meta-analyses, randomized controlled trials, clinical trials, clinical guidelines, and reviews. Also searched were the National Guideline Clearinghouse database, Essential Evidence Plus, UpToDate, the Cochrane database, and the Agency for Healthcare Research and Quality Clinical Guidelines and Evidence Reports. Search date: August 10, 2012.
