Am Fam Physician. 2020;102(5):298-304
Author disclosure: No relevant financial affiliations.
Thyroid nodules can be detected by ultrasonography in up to 68% of the general population. They are typically benign and are often discovered incidentally. The primary goal of thyroid nodule evaluation is to determine whether it is malignant. After thyroid ultrasonography has been performed, the next step is measurement of serum thyroid-stimulating hormone. If levels are low, a radionuclide thyroid uptake scan is indicated. Hyperfunctioning nodules are rarely malignant and do not require tissue sampling. Nonfunctioning nodules and nodules in a patient with a normal or high thyroid-stimulating hormone level may require fine-needle aspiration based on ultrasound characteristics and size. Nodules with suspicious features and solid hypoechoic nodules 1 cm or larger require aspiration. The Bethesda System (categories 1 through 6) is used to classify samples. Molecular testing can be used to guide treatment when aspiration yields an indeterminate result. Molecular testing detects mutations associated with thyroid cancer and can help inform decisions about surgical excision vs. continued ultrasound monitoring. Treatment of pregnant women with nonfunctioning thyroid nodules and of children with thyroid nodules is similar to that for nonpregnant adults, with the exception of molecular testing, which has not been validated in these populations. (Am Fam Physician. 2020;102(5):298–304. Copyright © 2020 American Academy of Family Physicians.)
New advances in molecular testing have changed the management of thyroid nodules. This article reviews the workup for thyroid nodules, including how to interpret ultrasound findings and fine-needle aspiration (FNA) cytopathology, and a comparison of new molecular testing modalities to determine the appropriate management strategy.
WHAT'S NEW ON THIS TOPIC
Over the past few years, molecular testing of fine-needle aspiration specimens has changed the way thyroid nodules with indeterminate cytology are managed. A benign pattern on molecular testing significantly decreases the risk of malignancy in indeterminate thyroid nodules. However, these nodules still require ultrasound surveillance.
There is growing evidence that low-risk micropapillary thyroid cancers smaller than 1 cm can be followed with observation as an alternative to immediate surgical excision.
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| Thyroid ultrasonography with a survey of the cervical lymph nodes should be performed in all patients with thyroid nodules.11,12 | C | Cross-sectional prevalence studies and expert opinion |
| The serum thyroid-stimulating hormone level should be measured during the initial evaluation of a thyroid nodule. If it is low, a radionuclide thyroid uptake scan should be performed.11,12 | C | Cross-sectional prevalence studies and expert opinion |
| Fine-needle aspiration is recommended for thyroid nodules 1 cm or larger that have a suspicious pattern on ultrasonography.11,12 | C | Cross-sectional prevalence studies and expert opinion |
| Before molecular testing is performed, patients should be counseled about the potential benefits and limitations of the test.11 | C | Expert opinion |
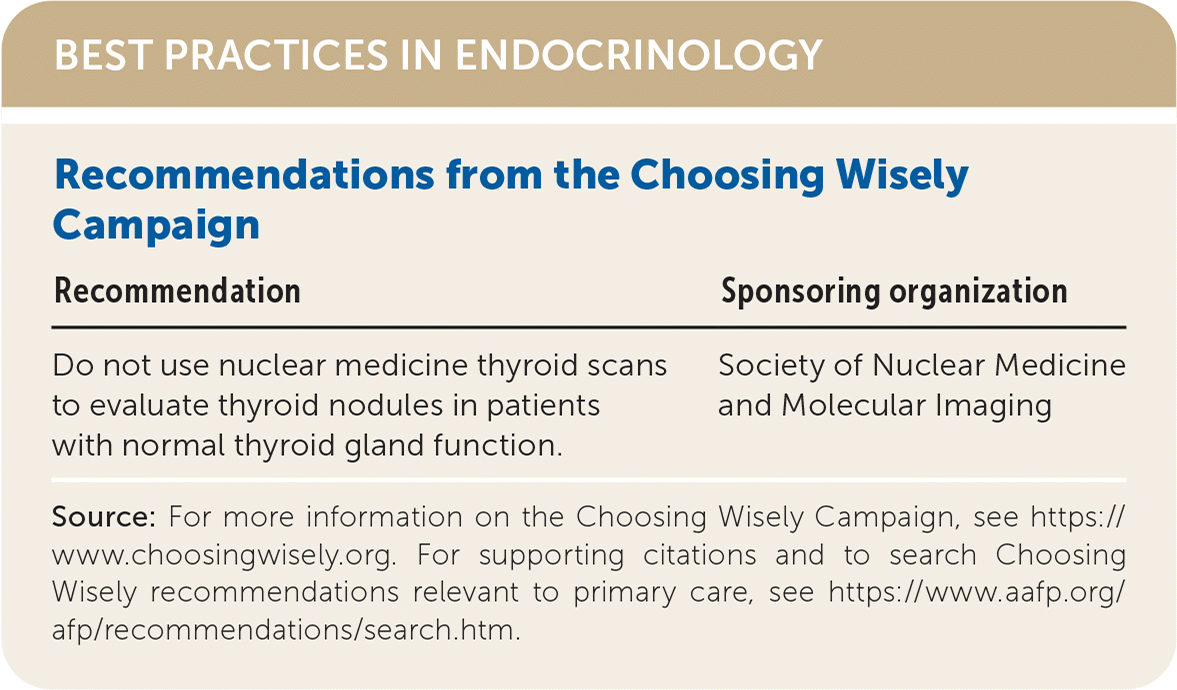
| Recommendation | Sponsoring organization |
|---|---|
| Do not use nuclear medicine thyroid scans to evaluate thyroid nodules in patients with normal thyroid gland function. | Society of Nuclear Medicine and Molecular Imaging |
Epidemiology
Thyroid nodules are more common in countries with iodine-deficient populations. The introduction of iodized salt in 1924 virtually eliminated iodine deficiency disorders in the United States.3 Thyroid nodules are four times more common in women than in men, and their prevalence increases with age and body mass index.4–6
Most thyroid nodules (90% to 95%) are benign.4,6 Risk factors for thyroid cancer include ionizing radiation (e.g., from cancer treatments, occupational exposure, or nuclear fallout, especially when the exposure occurs at a young age), rapid nodule growth, hoarseness, and a family history of thyroid cancer or cancer syndromes (e.g., multiple endocrine neoplasia type 2, familial adenomatous polyposis).7 Patients with Graves disease who have hypofunctioning nodules have a higher prevalence of papillary thyroid cancer (33% to 42%).8
The U.S. Preventive Services Task Force recommends against screening for thyroid cancer with neck palpation or ultrasonography.9 Screening results in overdiagnosis and overtreatment without improving patient outcomes. In South Korea, a widespread cancer screening program increased the thyroid cancer diagnosis rate 15-fold but did not change the mortality rate.10
Evaluation
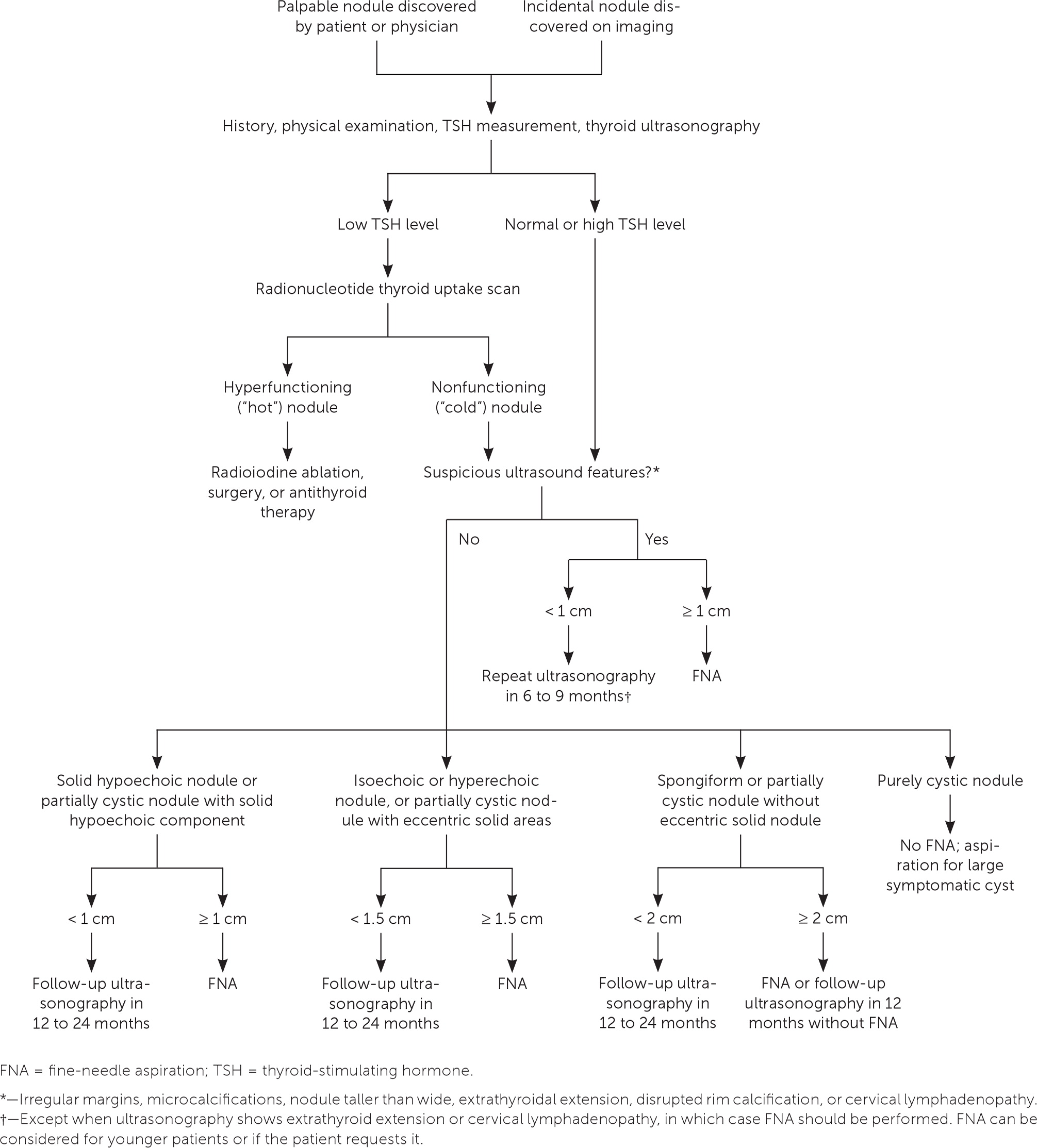
The first step in evaluating a thyroid nodule is to measure the thyroid-stimulating hormone (TSH) level and perform thyroid ultrasonography with a survey of the cervical lymph nodes.7,11,12 A normal or elevated TSH level indicates that the thyroid nodule is nonfunctioning; a low or suppressed TSH level suggests the diagnosis of primary hyperthyroidism, and a radionuclide thyroid uptake scan (technetium-99 or iodine-123) should be performed. Focal increased uptake in the region of the thyroid nodule is consistent with a hyperfunctioning or “hot” nodule. Hyperfunctioning nodules are unlikely to be malignant and do not require FNA. Nonfunctioning or “cold” nodules should be further evaluated with FNA if they meet clinical or ultrasound criteria.
Figure 1 suggests a management approach for thyroid nodules based on laboratory and ultrasound features.11 Nonfunctioning nodules have a 14% to 22% risk of malignancy.13 The risk of malignancy should be further stratified by ultrasound findings, which can be used to distinguish suspicious nodules that require further evaluation with FNA. According to the American College of Radiology, the American Thyroid Association, and the European Thyroid Association, ultrasound features that strongly suggest malignancy include hypoechoic echogenicity, solid composition, irregular margins, microcalcifications, height greater than width, extrathyroidal extension, disrupted rim calcification, and cervical lymph nodes with suspicious features.11,14,15 FNA should not be performed on nodules smaller than 1 cm.11 Micropapillary thyroid cancers typically have an indolent course. However, patients younger than 40 years may have more progressive disease.16 Most nodules smaller than 1 cm that have highly suspicious ultrasound features (with the exception of extrathyroidal extension and suspicious cervical lymph nodes) can be followed with close surveillance and repeat thyroid ultrasonography in six to nine months. FNA can be considered for younger patients or if the patient requests it.11 Conversely, pure cystic nodules are rarely malignant and do not require evaluation with FNA. Spongiform and predominantly cystic nodules also have very low risk of malignancy, and biopsy should be considered only if the nodule is 2 cm or larger.11 These nodules can also be followed with thyroid ultrasonography in 12 to 24 months without FNA.
FNA has a vital role in risk stratification of thyroid nodules.7 The American Thyroid Association recommends that FNA cytopathology be reported using the six Bethesda System diagnostic categories11,17 (Table 117). Approximately 25% of thyroid FNA samples are classified as Bethesda category 3 or 4, which are considered cytologically indeterminate18 and have a malignancy risk of 5% to 30%.17 Previously, the usual practice was to order diagnostic lobectomy in patients with these types of nodules. However, about 80% were ultimately found to be benign18; hence, surgical lobectomy is no longer considered ideal for all cytologically indeterminate nodules.
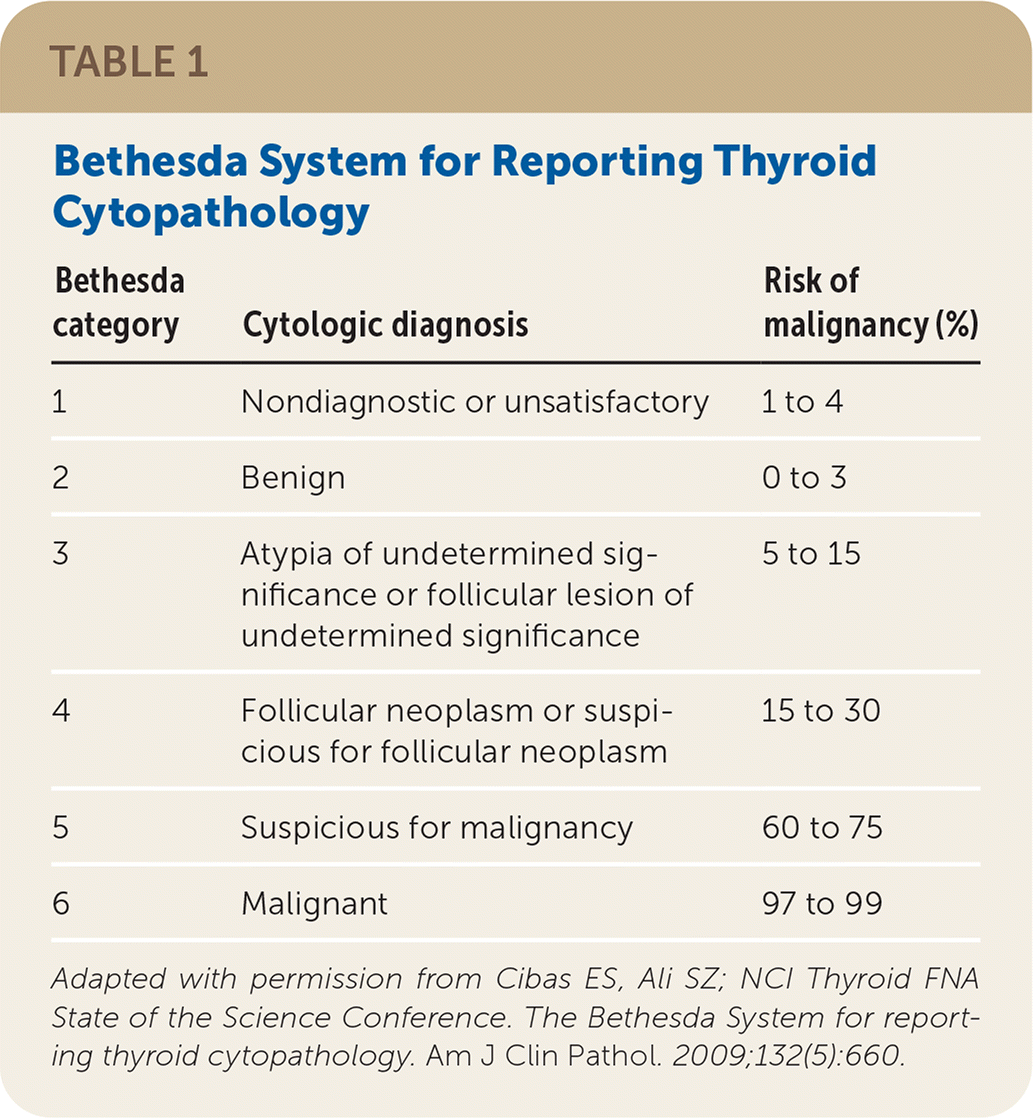
| Bethesda category | Cytologic diagnosis | Risk of malignancy (%) |
|---|---|---|
| 1 | Nondiagnostic or unsatisfactory | 1 to 4 |
| 2 | Benign | 0 to 3 |
| 3 | Atypia of undetermined significance or follicular lesion of undetermined significance | 5 to 15 |
| 4 | Follicular neoplasm or suspicious for follicular neoplasm | 15 to 30 |
| 5 | Suspicious for malignancy | 60 to 75 |
| 6 | Malignant | 97 to 99 |
Over the past few years, molecular testing of FNA specimens has changed the way thyroid nodules with indeterminate cytology are managed.19 Several molecular tests are commercially available, and understanding their methodology and performance is paramount in selecting the appropriate test (Table 2).17,20–24 However, many clinicians may not have a choice because most centers offer only one. There has been a trend toward performing reflex molecular testing on cytologically indeterminate thyroid nodules even when testing is not directly ordered by physicians. The result is that primary care physicians often receive molecular test results when they order FNA; therefore, it is important to understand how to interpret these results.
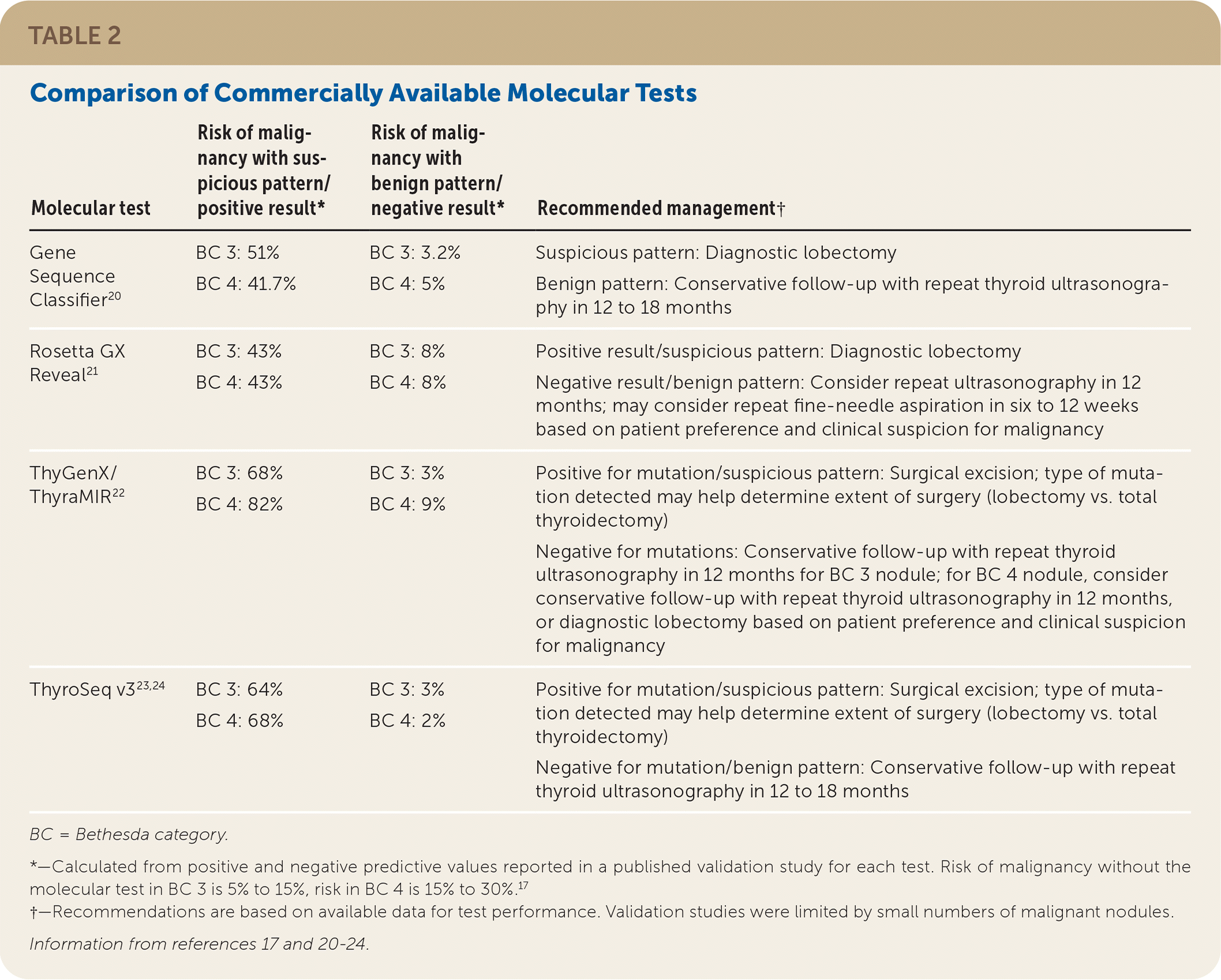
| Molecular test | Risk of malignancy with suspicious pattern/positive result* | Risk of malignancy with benign pattern/negative result* | Recommended management† |
|---|---|---|---|
| Gene Sequence Classifier20 | BC 3: 51% BC 4: 41.7% | BC 3: 3.2% BC 4: 5% | Suspicious pattern: Diagnostic lobectomy Benign pattern: Conservative follow-up with repeat thyroid ultrasonography in 12 to 18 months |
| Rosetta GX Reveal21 | BC 3: 43% BC 4: 43% | BC 3: 8% BC 4: 8% | Positive result/suspicious pattern: Diagnostic lobectomy Negative result/benign pattern: Consider repeat ultrasonography in 12 months; may consider repeat fine-needle aspiration in six to 12 weeks based on patient preference and clinical suspicion for malignancy |
| ThyGenX/ThyraMIR22 | BC 3: 68% BC 4: 82% | BC 3: 3% BC 4: 9% | Positive for mutation/suspicious pattern: Surgical excision; type of mutation detected may help determine extent of surgery (lobectomy vs. total thyroidectomy) Negative for mutations: Conservative follow-up with repeat thyroid ultrasonography in 12 months for BC 3 nodule; for BC 4 nodule, consider conservative follow-up with repeat thyroid ultrasonography in 12 months, or diagnostic lobectomy based on patient preference and clinical suspicion for malignancy |
| ThyroSeq v323,24 | BC 3: 64% BC 4: 68% | BC 3: 3% BC 4: 2% | Positive for mutation/suspicious pattern: Surgical excision; type of mutation detected may help determine extent of surgery (lobectomy vs. total thyroidectomy) Negative for mutation/benign pattern: Conservative follow-up with repeat thyroid ultrasonography in 12 to 18 months |
The American Thyroid Association recommends that after consideration of clinical and ultrasound features, molecular testing be used to further risk-stratify indeterminate thyroid nodules.11 The patient should be counseled about the potential benefits and limitations of molecular testing, and molecular testing should be ordered only after informed consent has been obtained. Validation studies on molecular tests are limited because of the small number of malignant nodules in these studies and insufficient long-term follow-up for benign nodules. In addition, the impact of suspicious ultrasound patterns and high-risk clinical features on the performance and interpretation of molecular tests has not been studied. Therefore, even though a benign pattern on molecular testing significantly decreases the risk of malignancy in indeterminate thyroid nodules, ultrasound surveillance is still required. Consultation with an endocrinologist may be helpful if there is uncertainty about how to interpret a molecular test result.
Treatment and Follow-up
Radioactive iodine ablation should be considered for hyperfunctioning thyroid nodules. If this procedure cannot be performed because of a contraindication or patient preference, hyperthyroidism should be treated with an antithyroid drug. To avoid lifelong antithyroid therapy, surgery should be considered after the patient is euthyroid. Figure 2 reviews the management of thyroid nodules after FNA. If cytology and/or molecular test results show malignancy (Bethesda category 6) or suspicion for malignancy (Bethesda category 5, or category 3 or 4 with suspicious molecular test results), surgical referral is recommended to remove the affected thyroid lobe or the entire thyroid gland.11,19
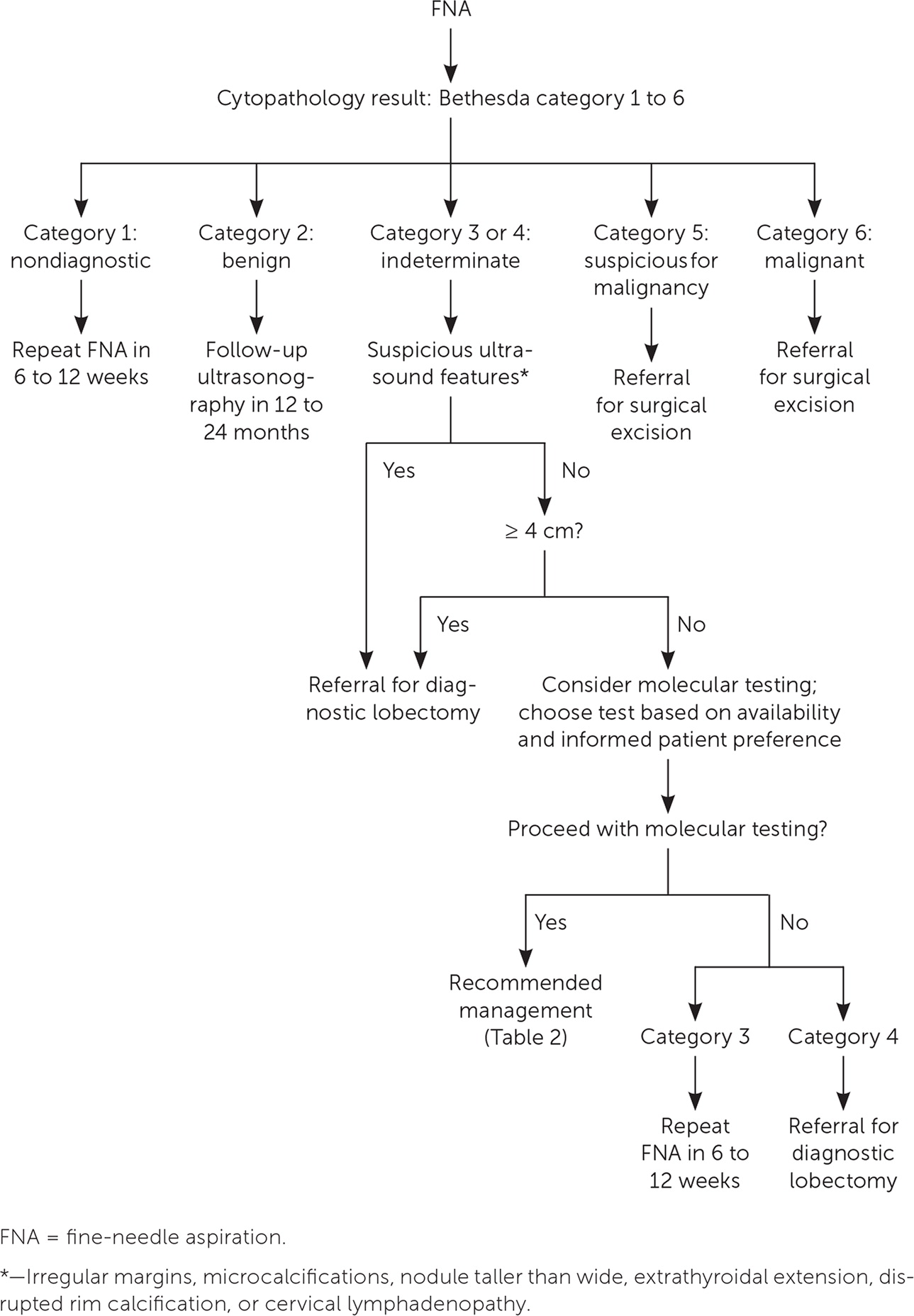
There is growing evidence that low-risk micropapillary thyroid cancers smaller than 1 cm can be followed with observation as an alternative to surgical excision. An observational study of Japanese patients who were followed with serial thyroid ultrasonography for an average of 75 months showed that low-risk micropapillary thyroid cancers (without extrathyroidal extension, metastatic cervical lymph nodes, or distant metastases) usually have an indolent course, without distant metastases or death.16 However, patients younger than 40 years had significantly higher rates of new cervical lymph node detection and tumor growth compared with those older than 60 years. Therefore, active surveillance can be considered in patients with low-risk micropapillary thyroid cancer as an alternative to lobectomy.11 It is reasonable to consult an endocrinologist when formulating an individualized management plan for these patients.
Benign nodules (Bethesda category 2, or category 3 or 4 with benign molecular test results) should be followed with repeat ultrasonography 12 to 24 months after the initial FNA, based on the ultrasound characteristics of the nodules (Figure 2). If the nodules have not grown significantly, the interval may be extended to three to five years.25 An increase of more than 50% in nodule volume or 20% in at least two nodule dimensions is considered clinically significant.11 Nodules showing significant growth on follow-up thyroid ultrasonography should undergo repeat FNA or serial ultrasonography, based on the suspicion for malignancy.11 If molecular testing is not performed, patients with category 4 nodules should be referred for diagnostic lobectomy, whereas those with category 3 nodules can undergo repeat FNA.11 Repeat FNA should be performed for most nodules with a nondiagnostic cytology result (Bethesda category 1). If the nodule has highly suspicious ultrasound features or clinical risk factors, surgical excision should be considered for definitive diagnosis.
There have been reports of a higher prevalence of malignancy and reduced diagnostic accuracy of FNA in nodules 4 cm or larger, but this has not translated into higher mortality risk in patients who are followed without surgery.26–28 It is not clear whether long-term follow-up of cytologically benign nodules 4 cm or larger can be recommended. Even if ultrasound surveillance is not indicated based on benign cytology, larger nodules should be monitored for growth that could result in compressive symptoms. The American Thyroid Association recommends follow-up with repeat ultrasonography at 12 to 24 months for cytologically benign nodules with low- to intermediate-suspicion ultrasound features.11 Hence, it is reasonable to reevaluate larger thyroid nodules that were benign on FNA with follow-up ultrasonography at 12 to 24 months. Primary care physicians should have a low threshold for surgical referral if the nodule shows significant growth or if follow-up ultrasonography demonstrates a new suspicious pattern. Recurrent cystic nodules with benign histology may be removed surgically or percutaneously injected with ethanol if they are symptomatic.11 Solid nodules that are benign on repeat FNA may be followed with ultrasonography or removed surgically, depending on symptoms; however, repeat FNA is not required, even if the nodule shows growth.11 Studies of levothyroxine suppression in benign nodules have shown some reduction in nodule size,29 but this treatment is not recommended because the potential harm of thyrotoxicosis outweighs the benefit.11
Special Populations
PREGNANT WOMEN
Pregnant women may have thyroid nodules that grow in size. There is no evidence that thyroid nodules found during pregnancy have an increased risk of malignancy.11 Nonfunctioning thyroid nodules in pregnant women can be managed in the same way as those in nonpregnant women, with the exception of molecular testing, which has not been validated in this population.11 Pregnant women with hyperfunctioning thyroid nodules can be treated with antithyroid medications, but radionuclide thyroid uptake scans must be deferred until after pregnancy and lactation.11
PATIENTS WITH MULTIPLE NODULES
Because each thyroid nodule carries an independent risk of malignancy, patients with multiple nodules may require FNA for more than one nodule. Nodules with highly suspicious ultrasound features should be preferentially biopsied. A radionuclide thyroid uptake scan can be considered in patients with a low to low-normal TSH level to identify target nodules for FNA.
CHILDREN
Thyroid nodules in children are rare, but they carry a greater risk of malignancy than those in adults (22% to 26%).30 The evaluation and treatment are similar to those for adults; however, because thyroid volume increases with age, ultrasound features rather than size alone should be used to identify nodules that require FNA in children.30 Molecular testing of FNA specimens has not been validated in children, so surgery is recommended for those with indeterminate results.30
This article updates previous articles on this topic by Knox25 and by Welker and Orlov.31
Data Sources: We searched PubMed for articles published since 2010 using the key terms thyroid nodule, thyroid epidemiology, thyroid nodule management, thyroid nodules in children, and thyroid nodules in pregnancy. The search included meta-analyses, randomized controlled trials, clinical trials, reviews, and guidelines from the American Thyroid Association. An evidence summary from Essential Evidence Plus was also reviewed and relevant studies referenced. Search dates: September 13, September 30, and October 7, 2019, and April 19, 2020.