
Am Fam Physician. 2023;108(1):93-94
Author disclosure: No relevant financial relationships.
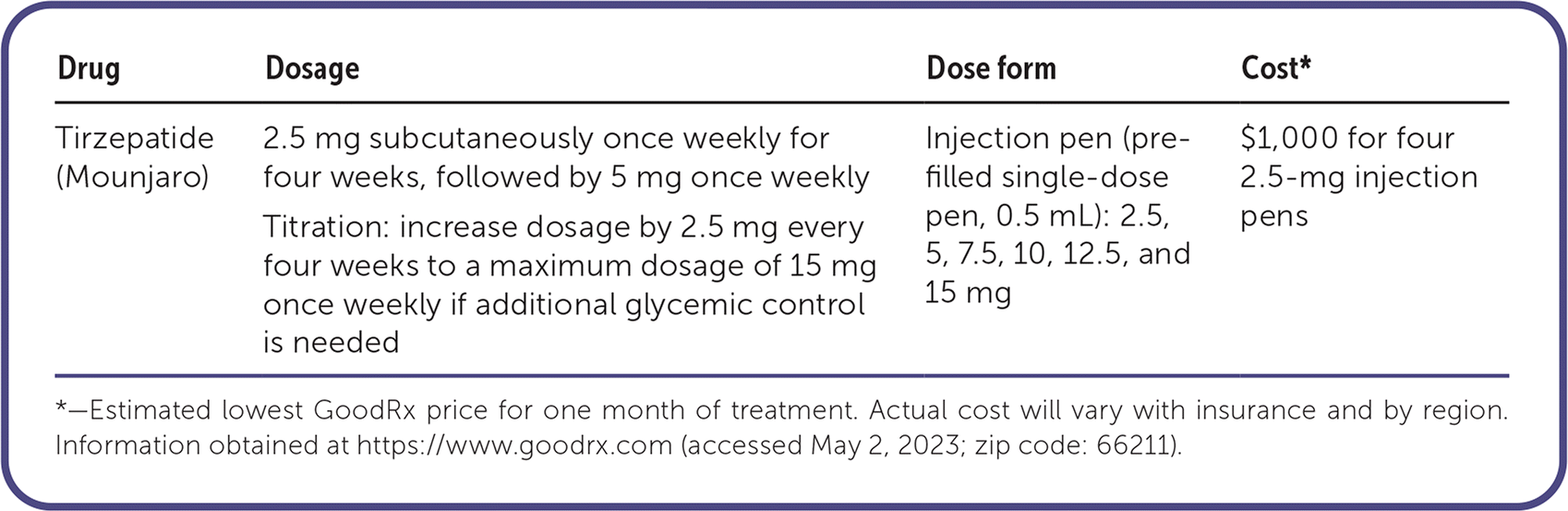
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Tirzepatide (Mounjaro) | 2.5 mg subcutaneously once weekly for four weeks, followed by 5 mg once weekly | Injection pen (pre-filled single-dose pen, 0.5 mL): 2.5, 5, 7.5, 10, 12.5, and 15 mg | $1,000 for four 2.5-mg injection pens |
| Titration: increase dosage by 2.5 mg every four weeks to a maximum dosage of 15 mg once weekly if additional glycemic control is needed |
Tirzepatide (Mounjaro) is labeled for the treatment of type 2 diabetes mellitus in adults as an adjunct to lifestyle modifications. It is the first agonist that targets both glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide receptors.1 This dual effect is hypothesized to impact glycemic control and weight loss more than GLP-1 receptor agonism alone due in part to the prevalence of glucose-dependent insulinotropic polypeptide receptors in adipose tissue and the brain.
Safety
Tirzepatide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma and in patients with multiple endocrine neoplasia syndrome type 2. Rarely, acute pancreatitis has been associated with the use of tirzepatide and other GLP-1 receptor agonists.2 There is also a small increased risk of gallbladder or biliary diseases that may be associated with GLP-1 receptor agonists; this risk could be dose, duration, or indication dependent.3 Tirzepatide may have a moderately higher risk of severe gastrointestinal adverse events compared with GLP-1 receptor agonists.4 Dose adjustment is not necessary in patients with renal or hepatic impairment.
Tirzepatide delays gastric emptying, which may reduce the effectiveness of oral contraceptives. If switching to a non–oral contraceptive method is not preferred by the patient, a barrier method is recommended for four weeks after initiation and dose escalation.
There are no data on the presence of tirzepatide in breast milk, and data for use in pregnant patients are insufficient to determine risk. There are no published data for the use of tirzepatide in children.
Tolerability
Adverse effects are similar to those of other GLP-1 receptor agonists. In a pool of placebo-controlled trials, gastrointestinal adverse effects occurred in more than 5% of patients taking tirzepatide. Nausea, vomiting, and diarrhea are more common during dose increases and generally improve over time. Tirzepatide does not increase the risk of hypoglycemia when used as monotherapy.
Effectiveness
Tirzepatide was studied in five trials at 5-, 10-, and 15-mg doses: as monotherapy, as an add-on to metformin, sulfonylureas, and sodium-glucose cotransporter-2 inhibitors; and as an add-on to basal insulin (with or without metformin). Tirzepatide produced significant reductions in A1C levels compared with placebo at all three doses (n = 718 in two trials).5,6 Tirzepatide has not been directly compared with semaglutide (Ozempic); however, in patients on stable dosages of metformin, tirzepatide will produce a 2.0% to 2.3% reduction in A1C levels from base line, which is 0.15 to 0.5 percentage points more than 1 mg of semaglutide.7 In addition, tirzepatide will reduce body weight by an average of 7.6 to 11.2 kg (16.8 to 24.7 lb) depending on the dose. This is 1.9 to 5.5 kg (4.2 to 12.1 lb) greater than treatment with semaglutide.7 In a 52-week study of 1,995 patients (modified intention-to-treat population) with type 2 diabetes taking up to three oral hypoglycemic agents and at an increased risk of cardiovascular events, tirzepatide was superior to insulin glargine at all three doses.8 Based on limited research, tirzepatide has not been shown to affect cardiovascular outcomes or reduce overall mortality compared with usual care.4
Price
Tirzepatide costs about $1,000 for a one-month supply (i.e., four 2.5-mg injection pens), in addition to the cost of other prescribed diabetes medications. Tirzepatide is not currently covered by Medicare Part D and often requires prior authorization before coverage by a commercial plan.
Simplicity
The starting dosage of tirzepatide is 2.5 mg once weekly and is titrated monthly as needed until a target dosage is reached. Tirzepatide is administered via an injection pen without a visible needle, which may help those patients uncomfortable with injection.
Bottom Line
Tirzepatide is effective in the treatment of type 2 diabetes in adults. It provides a higher percentage of weight loss compared with similar agents, which may be attributed to its unique mechanism of action. Tirzepatide does not replace first-line options for cardiorenal protection in patients with atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease. It may be considered for patients whose priorities are achievement and maintenance of weight loss goals. However, the high cost may limit its use.
Tirzepatide adds to the growing list of diabetes medications that may also facilitate weight loss and have been used off-label for weight loss in patients without type 2 diabetes.
