Am Fam Physician. 2024;109(3):277-278
Author disclosure: No relevant financial relationships.
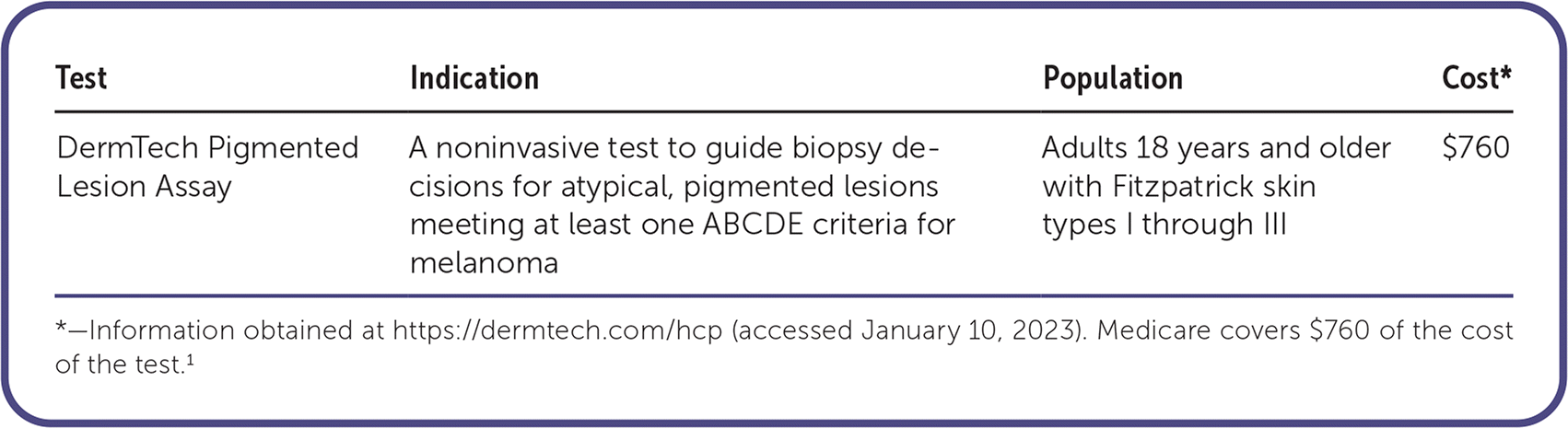
| Test | Indication | Population | Cost* |
|---|---|---|---|
| DermTech Pigmented Lesion Assay | A noninvasive test to guide biopsy decisions for atypical, pigmented lesions meeting at least one ABCDE criteria for melanoma | Adults 18 years and older with Fitzpatrick skin types I through III | $760 |
The DermTech Pigmented Lesion Assay (PLA) is a noninvasive test used for the detection of melanoma. Adhesive stickers are used to collect skin cells from the entire surface of suspicious melanocytic lesions that have one or more ABCDE criteria for melanoma. These stickers are analyzed for genomic markers associated with melanoma: RNA (LINC00518 and PRAME) and optional add-on DNA (TERT). When results are positive for one or more genomic markers, a biopsy is recommended. If results are negative for genomic markers, biopsy deferral and continued monitoring of the lesion are recommended.1 The PLA has not been approved by the U.S. Food and Drug Administration.1
Subscribe
From $180- Immediate, unlimited access to all AFP content
- More than 125 CME credits/year
- Print delivery available
Issue Access
$59.95- Immediate, unlimited access to this issue's content
- CME credits
- Print delivery available