Am Fam Physician. 2019;100(7):416-425
Related editorial: Common Misperceptions About Buprenorphine Prescribing for Opioid Use Disorder.
Patient information: See related handout on opioid use disorder.
Author disclosure: No relevant financial affiliations.
Opioid use disorder is highly prevalent and can be fatal. At least 2.1 million Americans 12 years and older had opioid use disorder in 2016, and approximately 47,000 Americans died from opioid overdoses in 2017. Opioid use disorder is a chronic relapsing condition, the treatment of which falls within the scope of practice of family physicians. With appropriate medication-assisted treatment, patients are more likely to enter full recovery. Methadone and buprenorphine are opioid agonists that reduce mortality, opioid use, and HIV and hepatitis C virus transmission while increasing treatment retention. Intramuscular naltrexone is not as well studied and is harder to initiate than opioid agonists because of the need to abstain for approximately one week before the first dose. However, among those who start naltrexone, it can reduce opioid use and craving. Choosing the correct medication for a given patient depends on patient preference, local availability of opioid treatment programs, anticipated effectiveness, and adverse effects. Discontinuation of pharmacotherapy increases the risk of relapse; therefore, patients should be encouraged to continue treatment indefinitely. Many patients with opioid use disorder are treated in primary care, where effective addiction treatment can be provided. Family physicians are ideally positioned to diagnose opioid use disorder, provide evidence-based treatment with buprenorphine or naltrexone, refer patients for methadone as appropriate, and lead the response to the current opioid crisis.
Opioid use disorder has reached epidemic proportions in the United States. At least 2.1 million Americans 12 years and older had opioid use disorder in 2016,1 and approximately 47,000 Americans died from opioid overdoses in 2017.2 People with opioid use disorder experience a loss of control over their use that can lead to physical disease and psychosocial disruptions, including unemployment, family disruption, and incarceration. Opioid use disorder can be fatal, with mortality rates 10-fold higher than in the general population.3 Death can occur from overdose, cardiovascular disease, or infectious diseases such as hepatitis C virus, HIV, and sepsis.
Opioid use disorder should be treated as a chronic condition with longitudinal, team-based, patient-centered care. Similar to type 2 diabetes mellitus or hypertension, opioid use disorder has genetic, environmental, and behavioral causes; the disorder responds best to long-term treatment with medication supplemented by behavior therapies. The American Academy of Family Physicians position paper on chronic pain and opioid misuse supports integrated chronic care with medication-assisted treatment, also known as medication addiction treatment, as part of a comprehensive primary care practice.4
Despite strong evidence that opioid use disorder is a chronic disease that responds to effective medical treatment, stigma against people who use illicit drugs can limit access to care. Furthermore, racial biases amplify negative consequences of substance use. For example, drug arrests are more likely to lead to treatment in white people and to incarceration in black people.5 To mitigate stigma, family physicians can emphasize the chronic disease model of addiction; avoid language such as abuser, dirty, and junkie; and participate in unconscious bias training.6,7
Risk Factors and Screening
Patients who have a history of trauma, adverse childhood events, and mental illness are at increased risk of opioid use disorder. Heritability of substance use disorder is estimated to be greater than 50%,8 and family history is a strong predictor of disease.
In 2008, the U.S. Preventive Services Task Force found insufficient evidence to assess the balance of benefits and harms of screening adolescents and adults for illicit drug use9; however, the task force recently announced a draft guideline that recommends screening all adults older than 18 years for illicit drug use.10 Some experts have also recommended screening all adults in primary care for opioid use disorder.11 Screening can be accomplished with a single question: How many times in the past year have you used an illegal drug or prescription medication for nonmedical reasons?12 A response of “at least once” is a positive screen and should prompt further assessment. Criteria from the Diagnostic and Statistical Manual of Mental Disorders, 5th ed., should then be used to make a diagnosis (Table 1).13

|
| Specify if: In early remission: After full criteria for opioid use disorder were previously met, none of the criteria for opioid use disorder have been met for at least 3 months but for less than 12 months (with the exception that Criterion A4, “Craving, or a strong desire or urge to use opioids,” may be met). In sustained remission: After full criteria for opioid use disorder were previously met, none of the criteria for opioid use disorder have been met at any time during a period of 12 months or longer (with the exception that Criterion A4, “Craving, or a strong desire or urge to use opioids,” may be met). |
| Specify if: On maintenance therapy: This additional specifier is used if the individual is taking a prescribed agonist medication such as methadone or buprenorphine and none of the criteria for opioid use disorder have been met for that class of medication (except tolerance to, or withdrawal from, the agonist). This category also applies to those individuals being maintained on a partial agonist, an agonist/antagonist, or a full antagonist such as oral naltrexone or depot naltrexone. In a controlled environment: This additional specifier is used if the individual is in an environment where access to opioids is restricted. |
| Coding based on current severity: Note for ICD-10-CM codes: If an opioid intoxication, opioid withdrawal, or another opioid-induced mental disorder is also present, do not use the codes below for opioid use disorder. Instead, the comorbid opioid use disorder is indicated in the 4th character of the opioid-induced disorder code (see the coding note for opioid intoxication, opioid withdrawal, or a specific opioid-induced mental disorder). For example, if there is comorbid opioid-induced depressive disorder and opioid use disorder, only the opioid-induced depressive disorder code is given, with the 4th character indicating whether the comorbid opioid use disorder is mild, moderate, or severe: F11.14 for mild opioid use disorder with opioid-induced depressive disorder or F11.24 for moderate or severe opioid use disorder with opioid-induced depressive disorder. Specify current severity: 305.50 (F11.10) Mild: Presence of 2–3 symptoms. 304.00 (F11.20) Moderate: Presence of 4–5 symptoms. 304.00 (F11.20) Severe: Presence of 6 or more symptoms. |
Medications for Opioid Use Disorder
With appropriate medications, patients are more likely to enter full recovery, which includes the ability to make self-directed choices, to contribute to family and community, and to reach one's full potential.14
Multiple medications are approved by the U.S. Food and Drug Administration for opioid use disorder (Table 2).15–21 Oral methadone, sublingual buprenorphine/naloxone (Suboxone), sublingual buprenorphine (Subutex), buprenorphine implants (Probuphine), intramuscular long-acting buprenorphine (Sublocade), and intramuscular long-acting naltrexone (Vivitrol) are effective treatments for opioid use disorder. Buprenorphine is approved by the U.S. Food and Drug Administration for patients 16 years and older, and methadone and naltrexone are approved for patients who are at least 18 years of age22 (Table 311,21–30).
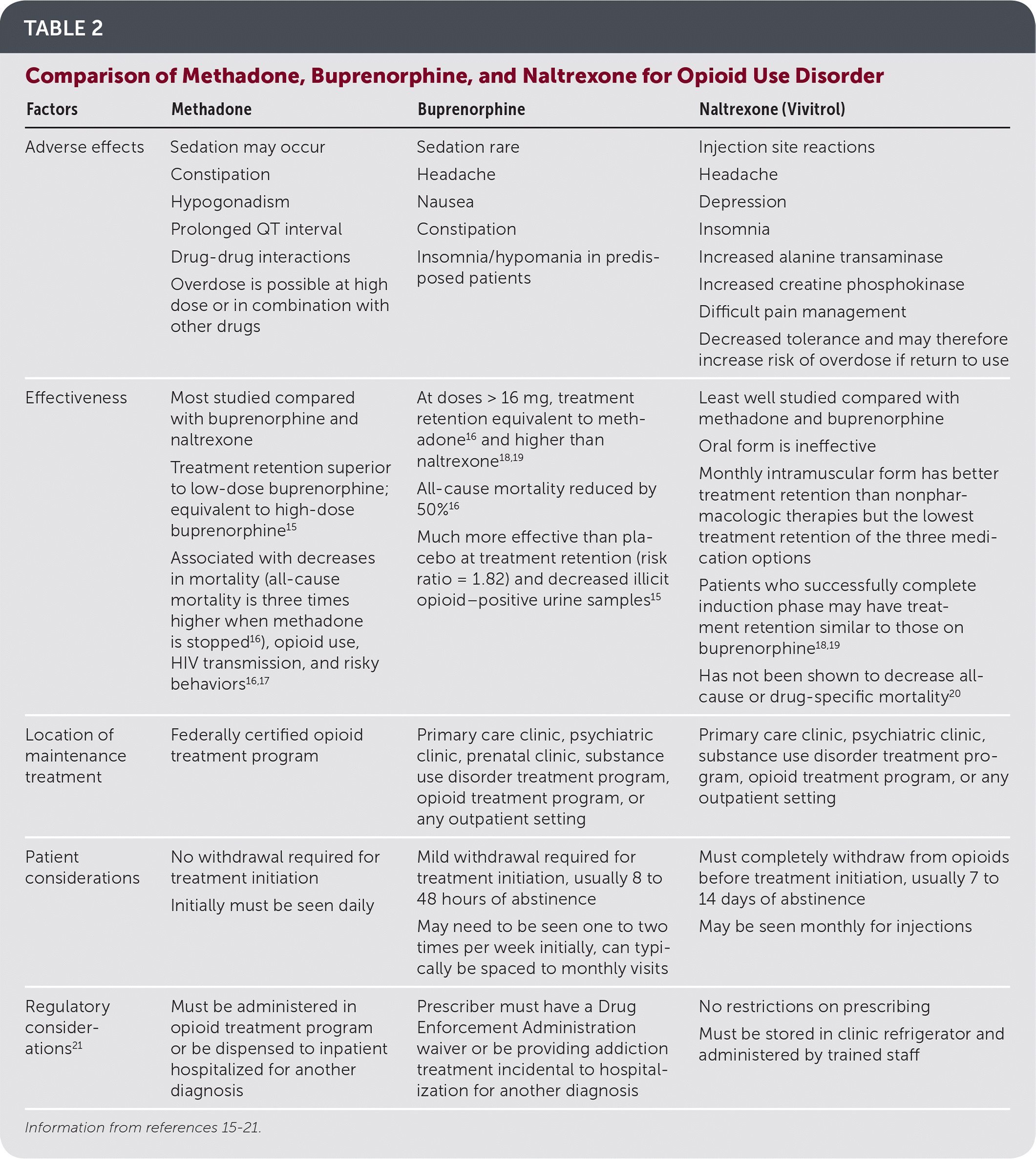
| Factors | Methadone | Buprenorphine | Naltrexone (Vivitrol) |
|---|---|---|---|
| Adverse effects | Sedation may occur Constipation Hypogonadism Prolonged QT interval Drug-drug interactions Overdose is possible at high dose or in combination with other drugs | Sedation rare Headache Nausea Constipation Insomnia/hypomania in predisposed patients | Injection site reactions Headache Depression Insomnia Increased alanine transaminase Increased creatine phosphokinase Difficult pain management Decreased tolerance and may therefore increase risk of overdose if return to use |
| Effectiveness | Most studied compared with buprenorphine and naltrexone Treatment retention superior to low-dose buprenorphine; equivalent to high-dose buprenorphine15 Associated with decreases in mortality (all-cause mortality is three times higher when methadone is stopped16), opioid use, HIV transmission, and risky behaviors16,17 | At doses > 16 mg, treatment retention equivalent to methadone16 and higher than naltrexone18,19 All-cause mortality reduced by 50%16 Much more effective than placebo at treatment retention (risk ratio = 1.82) and decreased illicit opioid–positive urine samples15 | Least well studied compared with methadone and buprenorphine Oral form is ineffective Monthly intramuscular form has better treatment retention than nonpharmacologic therapies but the lowest treatment retention of the three medication options Patients who successfully complete induction phase may have treatment retention similar to those on buprenorphine18,19 Has not been shown to decrease all-cause or drug-specific mortality20 |
| Location of maintenance treatment | Federally certified opioid treatment program | Primary care clinic, psychiatric clinic, prenatal clinic, substance use disorder treatment program, opioid treatment program, or any outpatient setting | Primary care clinic, psychiatric clinic, substance use disorder treatment program, opioid treatment program, or any outpatient setting |
| Patient considerations | No withdrawal required for treatment initiation Initially must be seen daily | Mild withdrawal required for treatment initiation, usually 8 to 48 hours of abstinence May need to be seen one to two times per week initially, can typically be spaced to monthly visits | Must completely withdraw from opioids before treatment initiation, usually 7 to 14 days of abstinence May be seen monthly for injections |
| Regulatory considerations21 | Must be administered in opioid treatment program or be dispensed to inpatient hospitalized for another diagnosis | Prescriber must have a Drug Enforcement Administration waiver or be providing addiction treatment incidental to hospitalization for another diagnosis | No restrictions on prescribing Must be stored in clinic refrigerator and administered by trained staff |
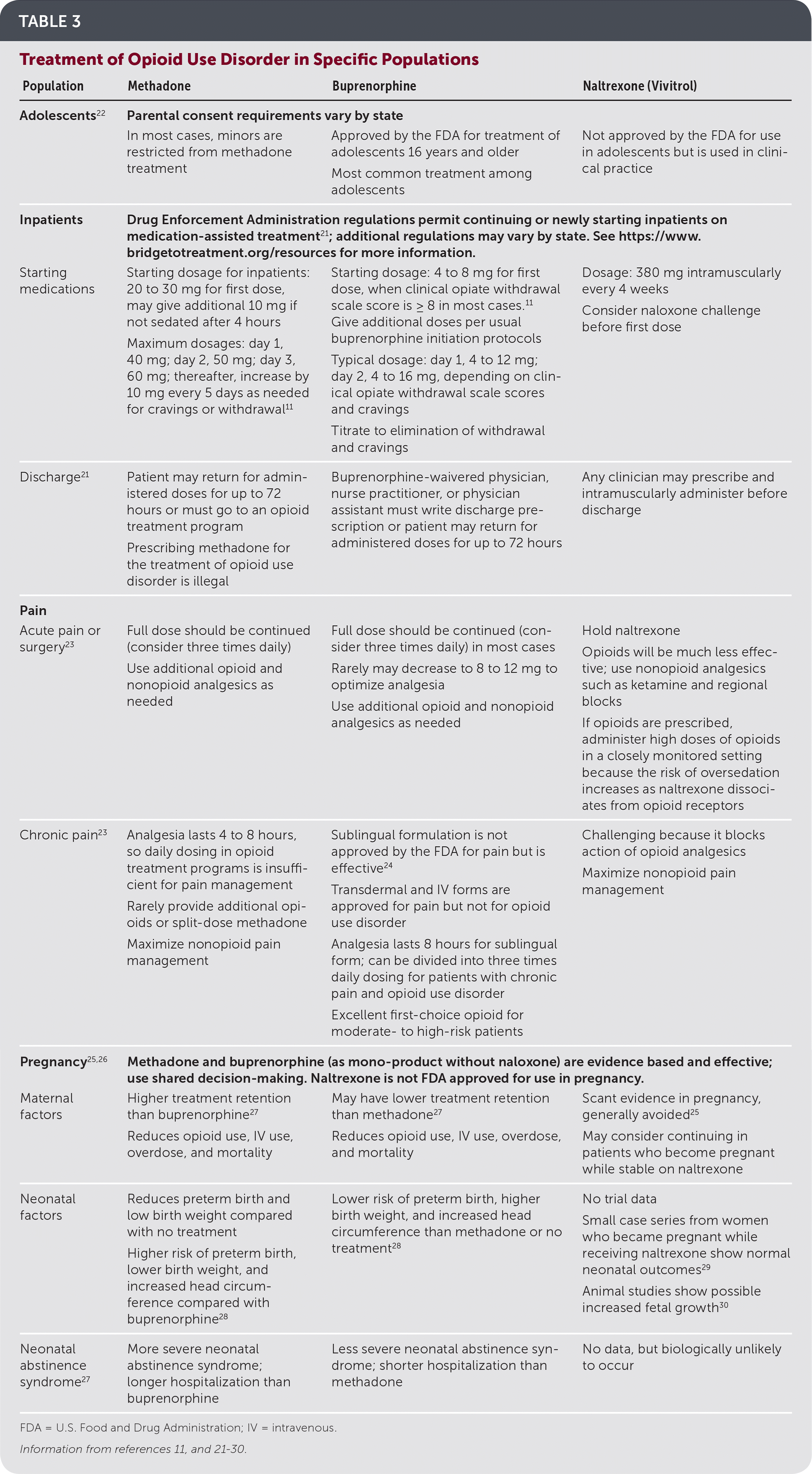
| Population | Methadone | Buprenorphine | Naltrexone (Vivitrol) |
|---|---|---|---|
| Adolescents22 | Parental consent requirements vary by state | ||
| In most cases, minors are restricted from methadone treatment | Approved by the FDA for treatment of adolescents 16 years and older Most common treatment among adolescents | Not approved by the FDA for use in adolescents but is used in clinical practice | |
| Inpatients | Drug Enforcement Administration regulations permit continuing or newly starting inpatients on medication-assisted treatment21 ; additional regulations may vary by state. See https://www.bridgetotreatment.org/resources for more information. | ||
| Starting medications | Starting dosage for inpatients: 20 to 30 mg for first dose, may give additional 10 mg if not sedated after 4 hours Maximum dosages: day 1, 40 mg; day 2, 50 mg; day 3, 60 mg; thereafter, increase by 10 mg every 5 days as needed for cravings or withdrawal11 | Starting dosage: 4 to 8 mg for first dose, when clinical opiate withdrawal scale score is ≥ 8 in most cases.11 Give additional doses per usualbuprenorphine initiation protocols Typical dosage: day 1, 4 to 12 mg; day 2, 4 to 16 mg, depending on clinical opiate withdrawal scale scores and cravings Titrate to elimination of withdrawal and cravings | Dosage: 380 mg intramuscularly every 4 weeks Consider naloxone challenge before first dose |
| Discharge21 | Patient may return for administered doses for up to 72 hours or must go to an opioid treatment program Prescribing methadone for the treatment of opioid use disorder is illegal | Buprenorphine-waivered physician, nurse practitioner, or physician assistant must write discharge prescription or patient may return for administered doses for up to 72 hours | Any clinician may prescribe and intramuscularly administer before discharge |
| Pain | |||
| Acute pain or surgery23 | Full dose should be continued (consider three times daily) Use additional opioid and nonopioid analgesics as needed | Full dose should be continued (consider three times daily) in most cases Rarely may decrease to 8 to 12 mg to optimize analgesia Use additional opioid and nonopioid analgesics as needed | Hold naltrexone Opioids will be much less effective; use nonopioid analgesics such as ketamine and regional blocks If opioids are prescribed, administer high doses of opioids in a closely monitored setting because the risk of oversedation increases as naltrexone dissociates from opioid receptors |
| Chronic pain23 | Analgesia lasts 4 to 8 hours, so daily dosing in opioid treatment programs is insufficient for pain management Rarely provide additional opioids or split-dose methadone Maximize nonopioid pain management | Sublingual formulation is not approved by the FDA for pain but is effective24 Transdermal and IV forms are approved for pain but not for opioid use disorder Analgesia lasts 8 hours for sublingual form; can be divided into three times daily dosing for patients with chronic pain and opioid use disorder Excellent first-choice opioid for moderate- to high-risk patients | Challenging because it blocks action of opioid analgesics Maximize nonopioid pain management |
| Pregnancy25,26 | Methadone and buprenorphine (as mono-product without naloxone) are evidence based and effective; use shared decision-making. Naltrexone is not FDA approved for use in pregnancy. | ||
| Maternal factors | Higher treatment retention than buprenorphine27 Reduces opioid use, IV use, overdose, and mortality | May have lower treatment retention than methadone27 Reduces opioid use, IV use, overdose, and mortality | Scant evidence in pregnancy, generally avoided25 May consider continuing in patients who become pregnant while stable on naltrexone |
| Neonatal factors | Reduces preterm birth and low birth weight compared with no treatment Higher risk of preterm birth, lower birth weight, and increased head circumference compared with buprenorphine28 | Lower risk of preterm birth, higher birth weight, and increased head circumference than methadone or no treatment28 | No trial data Small case series from women who became pregnant while receiving naltrexone show normal neonatal outcomes29 Animal studies show possible increased fetal growth30 |
| Neonatal abstinence syndrome27 | More severe neonatal abstinence syndrome; longer hospitalization than buprenorphine | Less severe neonatal abstinence syndrome; shorter hospitalization than methadone | No data, but biologically unlikely to occur |
Buprenorphine and methadone are safe and effective for use in pregnancy. They improve maternal and neonatal outcomes and are first-line treatments for opioid use disorder in pregnancy25 (Table 311,21–30). Family physicians can help stabilize families by integrating buprenorphine therapy with prenatal and postpartum care.
Medication selection should involve shared decision-making with patients and depends on effectiveness, adverse effects, patient preference, and availability (Table 215–21). Buprenorphine, methadone, and naltrexone work by reducing cravings and preventing intoxication if the patient resumes opioid use. Additionally, buprenorphine and methadone treat and prevent withdrawal and can help treat pain.
Medically assisted withdrawal, or detoxification, has high relapse rates, with more than 90% of patients returning to use within one month.31 Medications for addiction are most effective when prescribed as long-term, often lifelong, therapy. Patients should be discouraged from tapering off medication without a compelling cause.11
Long-term pharmacotherapy for opioid use disorder doubles the rate of abstinence relative to behavior therapy alone.32 Stand-alone behavior therapy is reserved for patients who decline medications. Adding behavior therapy to buprenorphine may not improve patient outcomes, with some studies showing benefit and others showing no improvement in treatment retention or illicit opioid use.33,34 Given this evidence, participation in behavior therapy should not be a precondition of receiving pharmacotherapy.11 Patients who engage in behavior therapy should seek therapists who support prescribing medication for opioid use disorder. Buprenorphine and naltrexone treatment can be integrated into family medicine clinics by using the Chronic Care Model.35 Behavioral health integration and group visits can enhance this model but are not required for successful outcomes.36,37 Studies have found no difference in outcomes for patients treated with buprenorphine in primary care vs. specialty addiction treatment.38 Table 4 shows a holistic approach to providing primary care for opioid use disorder.39

| Screen for HIV, hepatitis B virus, hepatitis C virus, sexually transmitted infections, and tuberculosis (at least annually for most patients) |
| Vaccinate against hepatitis A, hepatitis B, tetanus, diphtheria-pertussis, influenza, and pneumococcus |
| Aggressively manage cardiac risk factors including hypertension, lipid control, and smoking, particularly for people who also use stimulants or tobacco |
| Treat other comorbid substance use disorders, including tobacco and alcohol use disorders |
| Treat comorbid psychiatric disorders |
| Educate patients about safer injection practices and provide clean injection equipment |
| Offer pre-exposure HIV prophylaxis for patients who inject drugs or have other risk factors |
| Prescribe naloxone and discuss precautions such as test shots and using with friends |
| Offer methadone, buprenorphine, or naltrexone (Vivitrol) to all patients |
Methadone
MECHANISM
Methadone is a full agonist of the opioid receptor that prevents withdrawal, reduces cravings, and blunts the effects of other opioids.
EFFECTIVENESS
Methadone is the best studied medication for treating opioid use disorder and may be most effective for treatment retention.15 Its use is associated with a decrease in all-cause mortality of more than 50%,16 decreases in HIV risk behaviors,40 more than 50% decreased odds of hepatitis C virus incidence, and reduced use of nonprescribed opioids.17
REGULATORY ISSUES
Methadone can be prescribed for pain in primary care settings and administered for opioid use disorder in hospital settings; however, it is legal to prescribe outpatient methadone for opioid use disorder only in a federally certified opioid treatment program. To refer patients, consult the Substance Abuse and Mental Health Services Administration's opioid treatment program directory (https://dpt2.samhsa.gov/treatment/directory.aspx). Patients referred to these programs will initially receive daily directly observed dosing with frequent urine drug testing and behavior therapy. With increasing stability, patients may visit the program less often.
PATIENT SELECTION
Patients must have confirmed opioid use disorder and generally must be at least 18 years of age to enroll in an opioid treatment program. Patients with impaired liver function must also be monitored for oversedation, and electrocardiography should be performed on patients at risk for prolonged QT interval or patients receiving more than 120 mg per day of methadone because of concern for increased risk of torsades de pointes.41–43 Physicians should discuss with patients whether frequent visits to the program during the early months of maintenance therapy will help or hinder their stabilization.
DOSING CONSIDERATIONS
Dosing protocols are set by federal regulations. Initially, patients receive a low dosage, typically 20 to 40 mg per day. The dosage should be slowly increased until the medication prevents cravings while still minimizing sedation. Methadone is more effective at higher daily dosages (80 to 120 mg) than at moderate dosages (40 to 50 mg).43
Buprenorphine
A comprehensive discussion of buprenorphine can be found in a recent American Family Physician article.44
MECHANISM
Buprenorphine is a partial agonist of the opioid receptor. At increasing doses, buprenorphine reaches a ceiling effect. This minimizes sedation, euphoria, and respiratory depression. It is therefore rare for patients to overdose on buprenorphine, with most overdoses occurring in children or in adults who combined buprenorphine with benzodiazepines or large amounts of alcohol.45,46
If a patient's opioid receptors are occupied by a full agonist such as heroin or prescription opioids, taking buprenorphine displaces the full agonist from the receptor and replaces it with a partial agonist, reducing receptor activation. This can cause precipitated withdrawal. Therefore, patients should be in mild to moderate withdrawal before receiving buprenorphine.
Buprenorphine can be coformulated with naloxone, an opioid antagonist that is not orally or sublingually bioavailable. Naloxone has no effect on patients who take the medication as prescribed. It is added as an abuse deterrent; if injected or inhaled, the naloxone will be absorbed and may cause withdrawal.
EFFECTIVENESS
Buprenorphine and buprenorphine/naloxone are effective and can be prescribed in primary care clinics by appropriately trained clinicians. Buprenorphine, when provided in dosages of at least 16 mg per day, seems to be as effective as methadone at reducing illicit opioid use and for treatment retention.15
REGULATORY ISSUES
To prescribe buprenorphine for opioid use disorder, a physician must be board certified in addiction medicine or addiction psychiatry or complete an eight-hour training session to receive a waiver from the Drug Enforcement Administration. Nurse practitioners and physician assistants need 16 additional hours of training. In hospital settings, physicians without Drug Enforcement Administration waivers are allowed to administer buprenorphine to treat withdrawal and opioid use disorder.
Without directly observed dosing, diversion of buprenorphine is a risk; however, minimal risk of overdose and limited euphoria occur. Most patients who use illicit buprenorphine do so to treat their withdrawal symptoms and to quit opioids rather than to achieve euphoria.47
PATIENT SELECTION
Most patients with opioid use disorder are candidates for primary care prescription of buprenorphine. Previously, the U.S. Food and Drug Administration warned against prescribing buprenorphine to patients who used benzodiazepines or alcohol because of the elevated risk of overdose. This warning was recently removed because failing to treat opioid use disorder in the setting of alcohol and benzodiazepine use is riskier than treatment.48 Hepatotoxicity has been reported with buprenorphine use, and naloxone may have more potency in those with liver disease; therefore, patients with Child-Pugh scores of 7 or higher should be prescribed buprenorphine alone at a decreased dosage, and liver function tests should be monitored.11 Patients receiving chronic opioids can be transitioned to buprenorphine for pain management with good effect 49 (Table 311,21–30).
DOSING CONSIDERATIONS
Patients must be in mild to moderate withdrawal before initiating treatment. Medication initiation can safely occur at home.50 Over ensuing days, the patient's dose should be titrated up to eliminate withdrawal symptoms and to reduce cravings. Typical doses range from 12 mg to 24 mg; higher doses are associated with improved treatment retention and reduced illicit opioid use.11
Naltrexone
MECHANISM
Naltrexone is an antagonist of the opioid receptor. Upon binding, it provides opioid blockade, limiting the effect of other opioids and reducing cravings.51
EFFECTIVENESS
Oral naltrexone (Revia) is rarely effective for patients with opioid use disorder because of low treatment adherence.18 Intramuscular naltrexone reduces cravings and opioid use, but its effectiveness is limited by high drop-out rates during treatment induction. Patients who successfully initiate intramuscular naltrexone therapy appear to have rates of opioid abstinence similar to patients receiving buprenorphine.19 Unlike buprenorphine and methadone, naltrexone has not been shown to reduce the risk of mortality and overdose.20 Patients receiving naltrexone lose their opioid tolerance; therefore, a greater risk of overdose could occur if patients return to using opioids.52
REGULATORY ISSUES
Naltrexone can be prescribed by any physician. The medication must be stored in a refrigerator and administered by trained staff.
PATIENT SELECTION
Patients must abstain from opioids for seven to 14 days before initiating naltrexone therapy to avoid precipitated withdrawal. The intramuscular formulation has been associated with rare injection site reactions and transaminitis.11 In addition, patients receiving naltrexone will not respond to standard doses of opioids (Table 311,21–30). With severe pain, patients may require alternative medications, regional or general anesthesia, or high dosages of opioids. Naltrexone should be withheld before elective surgery, and patients should wear medical alert jewelry or carry a wallet card with information about the medication.
DOSING CONSIDERATIONS
Intramuscular naltrexone is administered in a dosage of 380 mg every four weeks. To confirm that the patient has completed opioid withdrawal, a naloxone challenge (i.e., 0.8 mg subcutaneously) may be administered before the first dose of naltrexone.11
Reducing Harm
All patients should be offered pharmacotherapy for opioid use disorder; however, some may not stop using, and others may return to use. It is important to discuss various harm reduction strategies with all patients who have a history of opioid use disorder. For more information, see American Family Physician's comprehensive article about caring for people who inject drugs.53
NALOXONE
The key medication for harm reduction in opioid use disorder is naloxone. Naloxone should be prescribed to any patient who receives chronic prescribed opioids (particularly in dosages greater than or equal to 50 morphine mg equivalents daily54), has a history of opioid use disorder, or uses illicit drugs.55,56 Nonmedical opioids, benzodiazepines, cocaine, and methamphetamine may be contaminated with fentanyl and other synthetic opioids, placing all people who use illicit drugs at risk of opioid overdose.57 Naloxone should be prescribed to friends or family who might witness an overdose. Most states have laws that allow prescribing to third parties; these laws protect prescribers and bystanders who administer naloxone.58 Intranasal formulations are easily administered by individuals with minimal training. Patients ty pically appreciate being offered naloxone and do not increase unsafe use patterns (e.g., taking extra doses, mixing with other drugs) after receiving the prescription.59 Visit https://www.prescribetoprevent.org for more information.
Data Sources: A PubMed search was completed using the search terms opioid use disorder treatment; buprenorphine; methadone; naltrexone; buprenorphine in pregnancy; methadone in pregnancy; naltrexone in pregnancy; pain buprenorphine; pain naltrexone; pain methadone; buprenorphine in hospital; methadone in hospital; naltrexone in hospital; buprenorphine adolescent; methadone adolescent; and naltrexone adolescent. In addition, we used Essential Evidence Plus, the Cochrane database, the DEA website, the CDC website, and the Substance Abuse and Mental Health Services Administration (SAMHSA) Treatment Improvement Protocol website. Search dates: April 15 to May 1, 2018.
Editor's Note: In April 2021, the Department of Health and Human Services (HHS) released new Buprenorphine Practice Guidelines in an effort to increase access to buprenorphine for the treatment of opioid use disorder. The new guidelines created an exemption from the required training component (8 hours for physicians; 24 hours for nurse practitioners and physician assistants) for qualified medical providers who want to treat 30 or fewer patients. Medical providers who are interested in prescribing buprenorphine still need to have a valid Drug Enforcement Administration (DEA) registration and a valid state license and are still required to complete the Notice of Intent (NOI). Once the Notice of Intent is approved, the medical provider can begin prescribing buprenorphine for the treatment of opioid use disorder. Medical providers who wish to treat more than 30 patients at any given time do still need to complete the mandatory training to have their patient limit increased. More information can be found here. HHS encourages medical providers new to prescribing buprenorphine to review SAMHSA’s Quick Start Guide and FDA prescribing guidelines.—Elizabeth Salisbury-Afshar, MD, Contributing Editor and Addiction Medicine representative to the AFP Editorial Advisory Board