
Am Fam Physician. 2023;108(6):562-573
Patient information: A related handout on psoriasis is available.
Author disclosure: No relevant financial relationships.
Psoriasis is an inflammatory skin and systemic disorder that affects 3.2% of the U.S. population, including 1% of children. It is an immune-mediated process triggered by an interplay of genetic, environmental, physical (e.g., skin trauma), and infectious factors. Associated comorbidities include cardiovascular disease, obesity, metabolic syndrome, diabetes mellitus, and inflammatory bowel disease. Psoriasis presents in various forms, including plaque, guttate, erythrodermic, pustular, inverse, nail, and psoriatic arthritis. The most common form is plaque psoriasis, which affects 90% of adults with psoriasis. Psoriasis is diagnosed clinically based on the presence of characteristic erythematous, scaly skin plaques in typical locations, with associated history and systemic symptoms. Treatment strategies are similar for most forms of psoriasis and based on body surface area involved. Topical corticosteroids, vitamin D analogues, and tazarotene are used to treat mild to moderate disease. Systemic treatment with nonbiologic and biologic agents and ultraviolet B phototherapy are used for moderate to severe disease, with the exception of apremilast, a systemic agent approved for mild psoriasis. Disease management is improved with maintaining ideal body weight, avoiding tobacco products, limiting alcohol, and practicing stress reduction techniques. The Psoriasis Area and Severity Index is a tool to assess severity and monitor treatment effectiveness over time. Special consideration is needed for treatment of children and patients who are pregnant, breastfeeding, or trying to conceive.
Psoriasis is an inflammatory skin and systemic disorder that affects 3.2% of the U.S. population, including 1% of children.1 In adults with psoriasis, 90% have plaque psoriasis.2 Up to 30% of adults with plaque psoriasis develop psoriatic arthritis, with a median onset of 10 to 11 years following the development of skin abnormalities.3 However, one prospective study showed that arthritic symptoms preceded skin findings in 19.4% of adults. In children, arthritis may precede skin findings by two to three years with bimodal age presentation at two to three years and 10 to 12 years.4,5
Pathophysiology
Psoriasis is a systemic immune-mediated disorder that involves an interplay between genetic predisposition and environmental stressors, including physical skin trauma and infectious factors. T cells create an inflammatory reaction by activating cytokines and growth factors that produce signals to leukocytes. This causes proliferation of keratinocytes that create plaques.6
Risk Factors
Individuals whose parents both have psoriasis have a 50% chance of developing the disease; this decreases to 16% if only one parent is affected.7 People with type 2 diabetes mellitus also have an increased risk of psoriasis based on a shared genetic loci.8 Inflammatory bowel disease is another risk factor.9
Modifiable risk factors for psoriasis include maintaining a healthy body weight, preventing metabolic syndrome, and avoiding tobacco and alcohol use.10–13 Lithium, beta blockers, angiotensin-converting enzyme inhibitors, and nonsteroidal anti-inflammatory drugs are associated with the development of psoriasis. Plaque development may occur 10 to 20 days after skin trauma, including tattoos, an effect known as the Koebner phenomenon.14
Although streptococcal infection is a trigger for the development of guttate and plaque psoriasis, a Cochrane review demonstrated insufficient evidence that antibiotic treatment of streptococcal infections shortens the duration of guttate psoriasis or prevents progression to plaque psoriasis.15
Clinical Presentation and Differential Diagnosis
Psoriasis is diagnosed clinically based on the presence of characteristic erythematous, scaly skin plaques in typical locations, with associated history and systemic symptoms. Subtypes of psoriasis include plaque, guttate, erythrodermic, pustular, and inverse. Patients may also develop nail psoriasis and psoriatic arthritis.
PLAQUE PSORIASIS
Plaque psoriasis can have a variety of appearances depending on an individual’s skin type (Figure 1). A silvery white appearance can occur in all skin types. In those with Fitzpatrick skin types 1 through 3 (always, usually, or sometimes sunburns), plaque psoriasis can be erythematous or salmon pink, and in those with types 4 through 6 (rarely sunburns, brown or black skin) it can be violaceous, red, or bluish.19 Plaques can also appear on the scalp.2
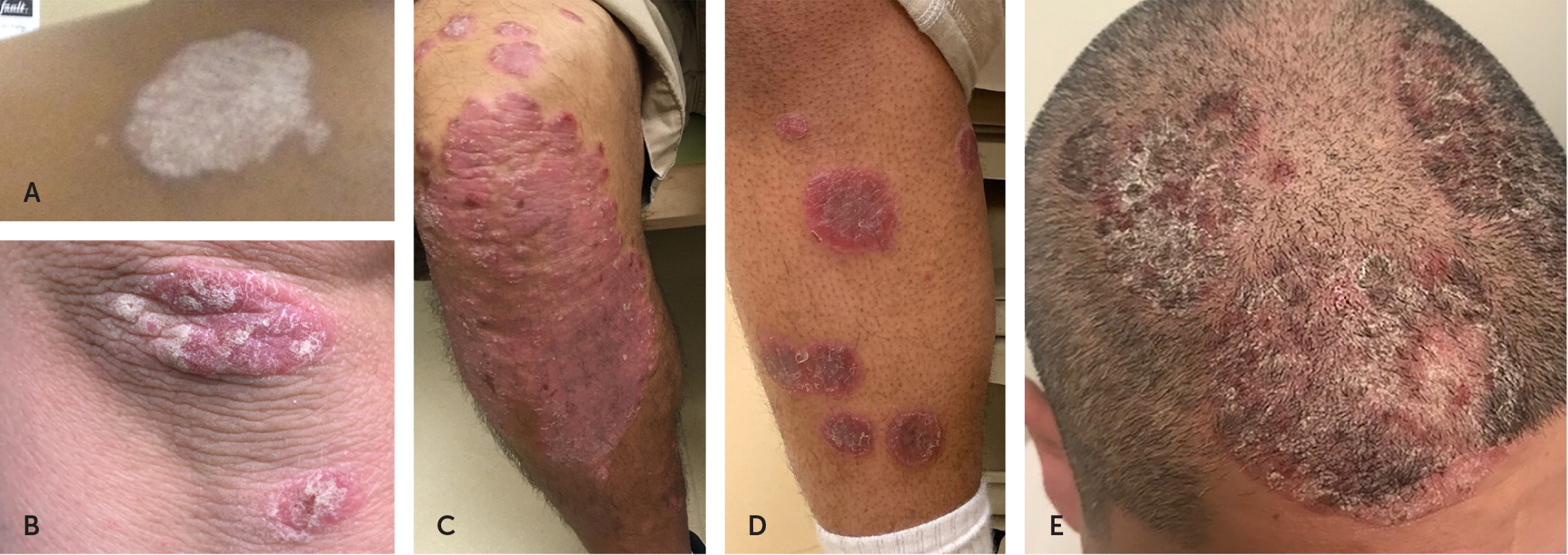
Several conditions can mimic plaque psoriasis. The most common is eczema, which lacks the silvery appearance and affects flexor, rather than extensor, surfaces. Tinea infections are distinguished from psoriasis by a central clearing. Squamous cell carcinoma tends to appear in sun-exposed skin, whereas psoriatic plaques tend to be on less sun-exposed skin.
GUTTATE PSORIASIS
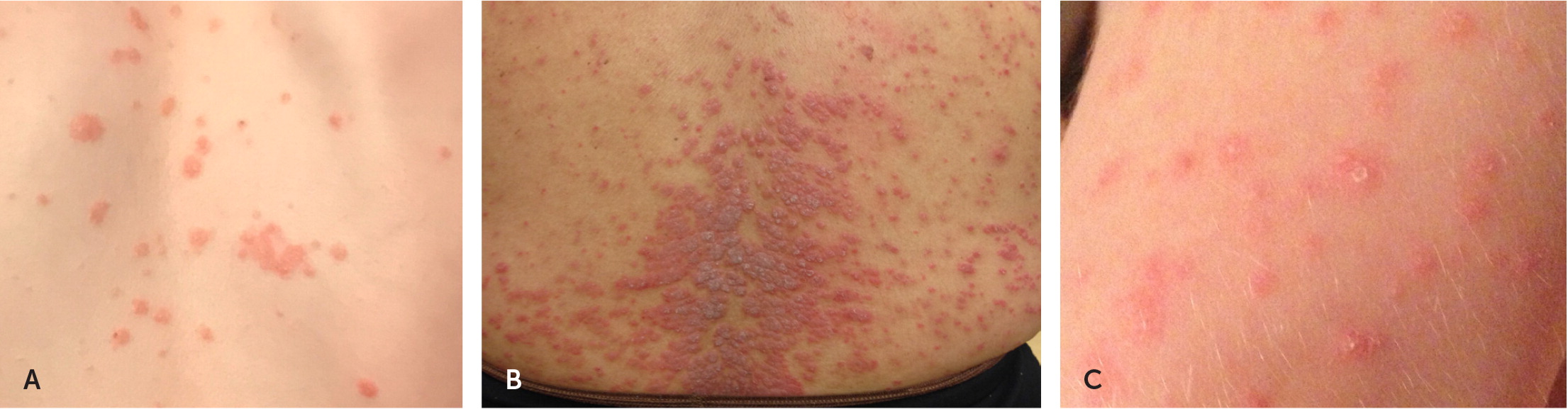
Guttate psoriasis presents as diffuse, small, erythematous, scaly papules and plaques20 (Figure 2). Pityriasis rosea can be distinguished from guttate psoriasis by the smaller lesions that form along skin cleavage lines on the trunk. Lesions from pityriasis rosea occur one to two weeks after a viral infection and resolve spontaneously. The differential diagnosis of guttate psoriasis includes the rash of secondary syphilis. This includes the presence of erythematous psoriasiform papules on palms and soles, which are spared in guttate psoriasis.
ERYTHRODERMIC PSORIASIS
Erythrodermic psoriasis presents with superficial desquamation and erythema (Figure 3), with or without exfoliation, and involves more than 75% of body surface area (BSA). Diagnosis is made clinically but can be verified histologically. Type 1 has a gradual progression over years, whereas type 2 develops abruptly and is a medical emergency.21 Common triggers include sunburn, alcohol use, HIV, lithium, and withdrawal of topical or oral corticosteroids.21
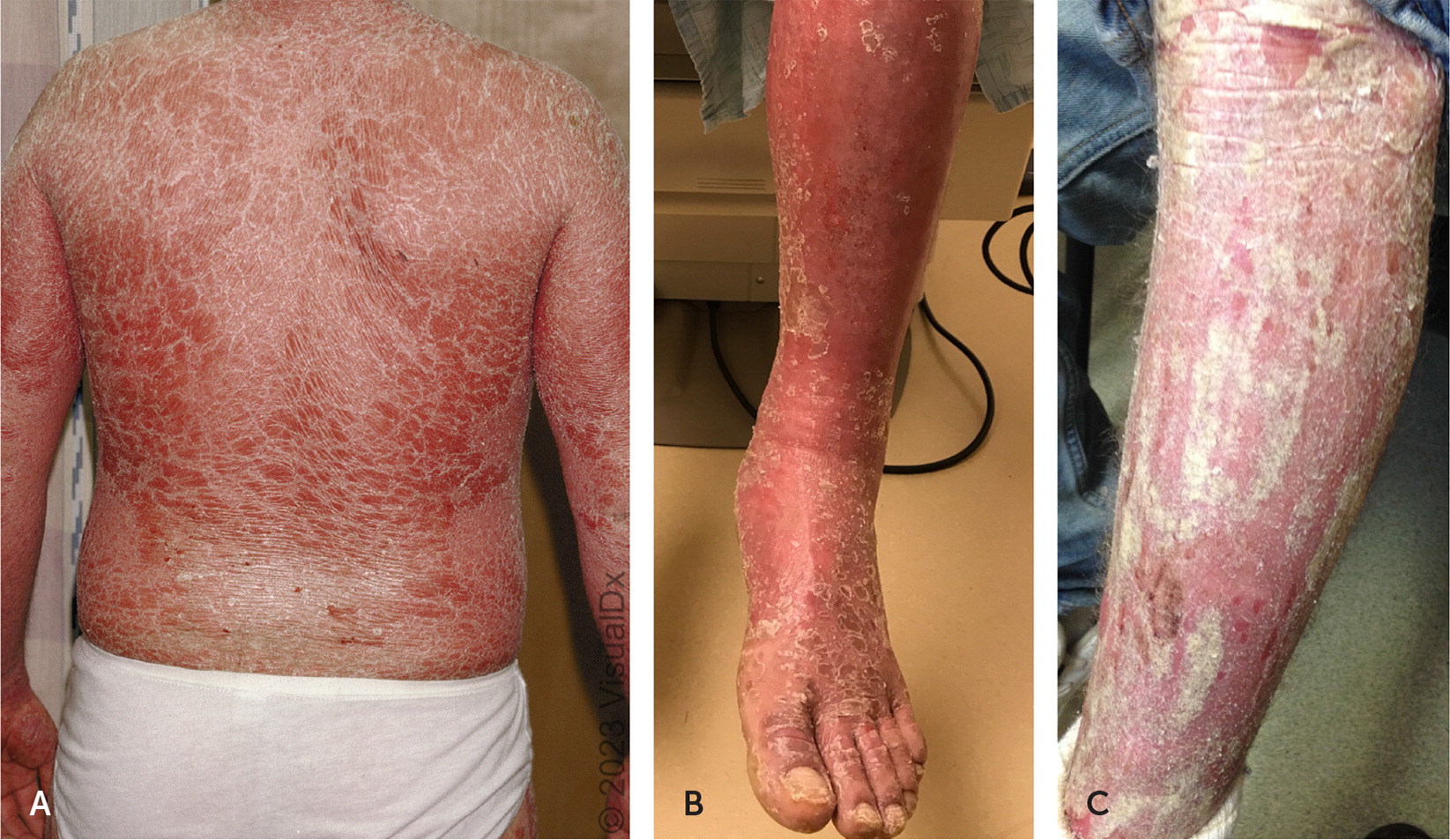
Although sometimes difficult to differentiate, patients with type 2 erythroderma are clinically unstable with diffuse erythema and no plaques. Patients may have hyper- or hypothermia, dehydration due to fluid loss, hypoalbuminemia, or sepsis because lesions often become superinfected with bacteria.21,22 The typical age of onset is 40 to 50 years.22 Differential diagnosis includes atopic dermatitis, fixed drug eruption, cutaneous sarcoidosis, and pityriasis rubra pilaris.
PUSTULAR PSORIASIS
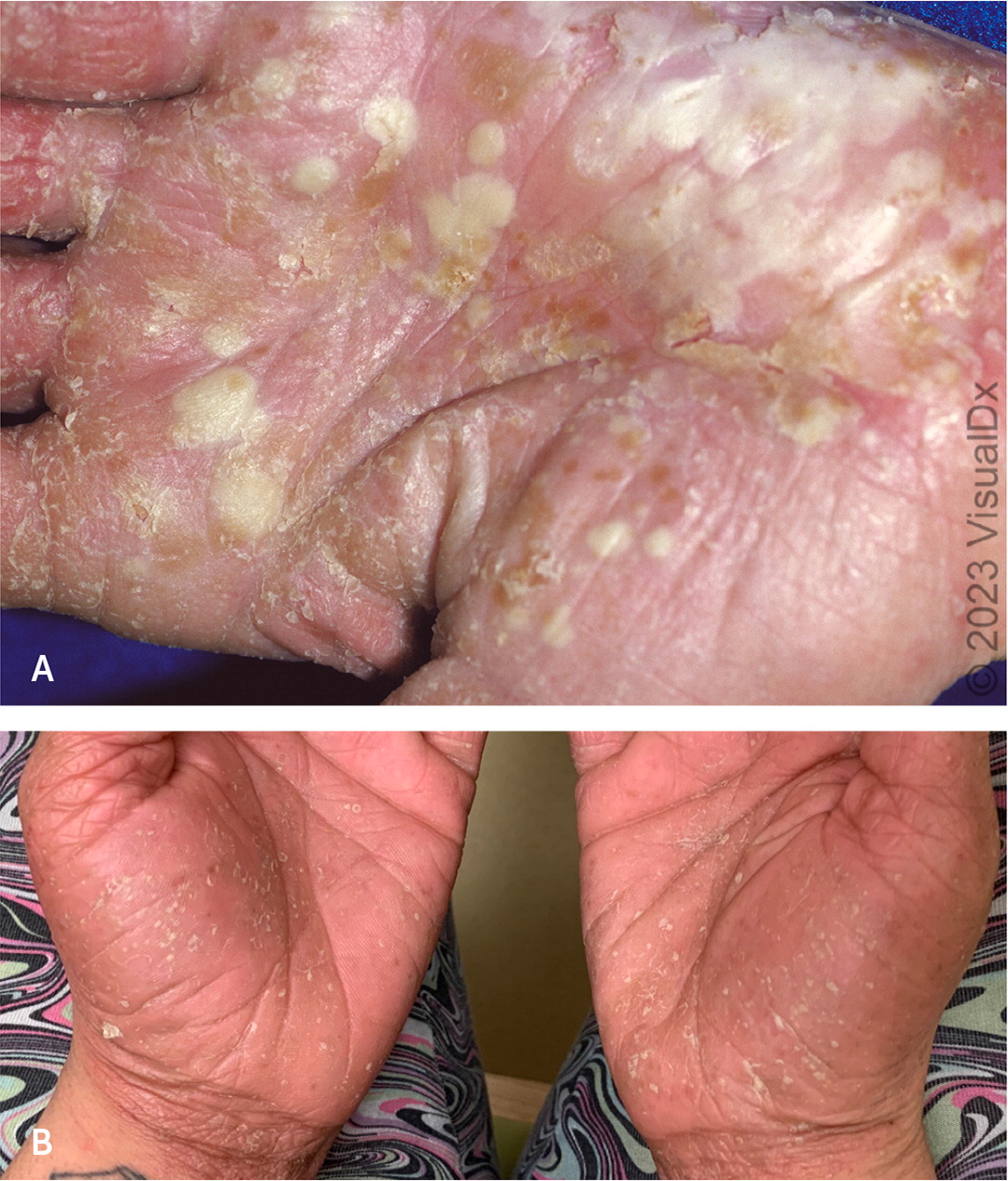
Pustular psoriasis presents with multiple pin-sized, sterile pustules on an erythematous base that can be generalized or only on the palms or soles23 (Figure 4). If a patient presents with generalized pustules, a biopsy of an intact pustule should be considered to rule out acute generalized exanthematous pustulosis, a disorder that develops rapidly (less than two weeks).24,25 In most cases (86%), pustular psoriasis is associated with starting or restarting an offending drug, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, or diltiazem.24,26 Other conditions included in the differential diagnosis are secondary infections of dermatitis, severe candidiasis, dermatitis herpetiformis, and diffuse impetigo.27
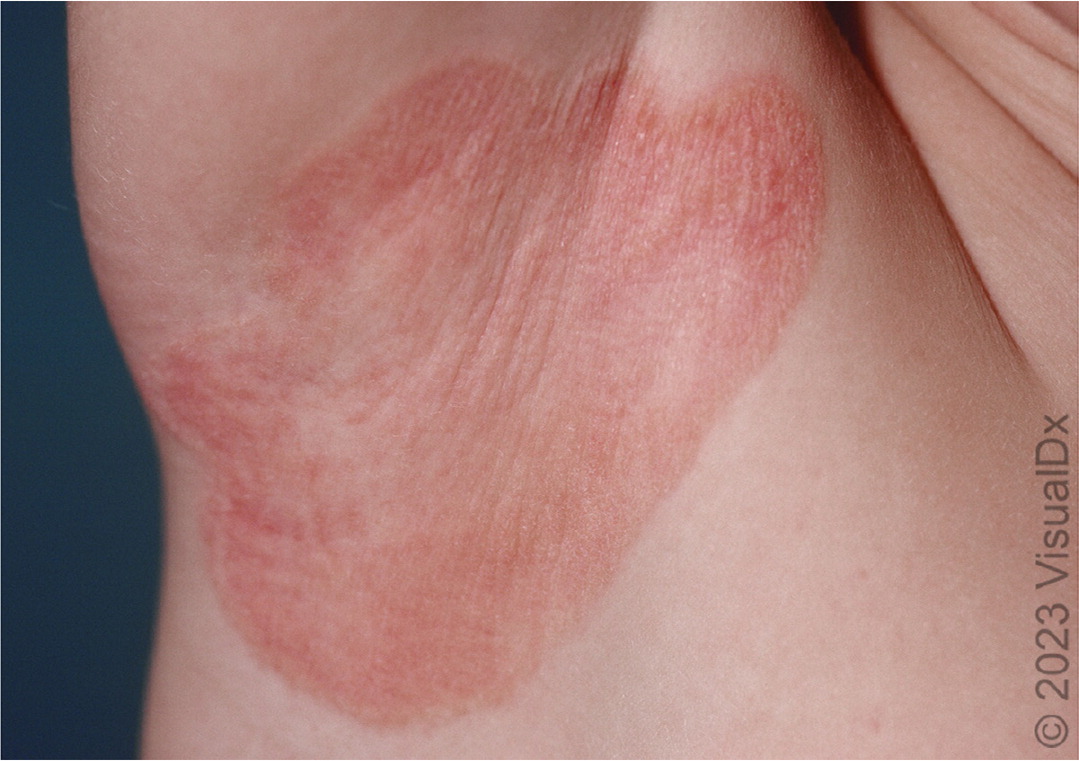
INVERSE PSORIASIS
Inverse psoriasis presents with well-demarcated erythematous lesions with minimal scale and is located within skin folds (Figure 5). Differential diagnosis includes fungal or bacterial infections of skin folds.
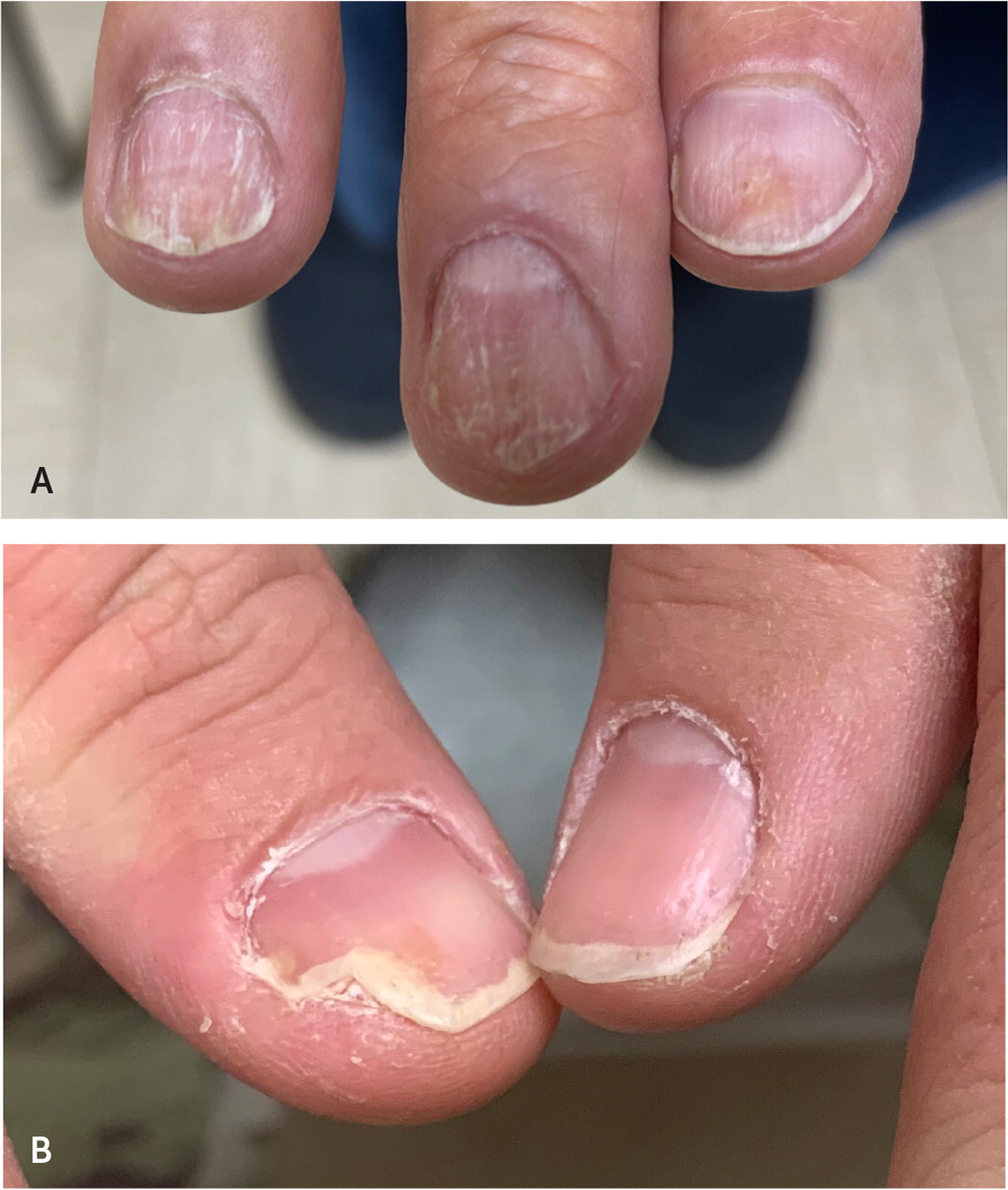
NAIL PSORIASIS
Nail psoriasis presents with pitting, distal onycholysis, oil-drop discoloration of the nail plate, and subungual hyper-keratosis28 (Figure 6). Patients with nail psoriasis have a higher risk of developing psoriatic arthritis.29 Periodic acid–Schiff stain, fungal cultures, or a potassium hydroxide preparation can help distinguish nail psoriasis from onychomycosis.
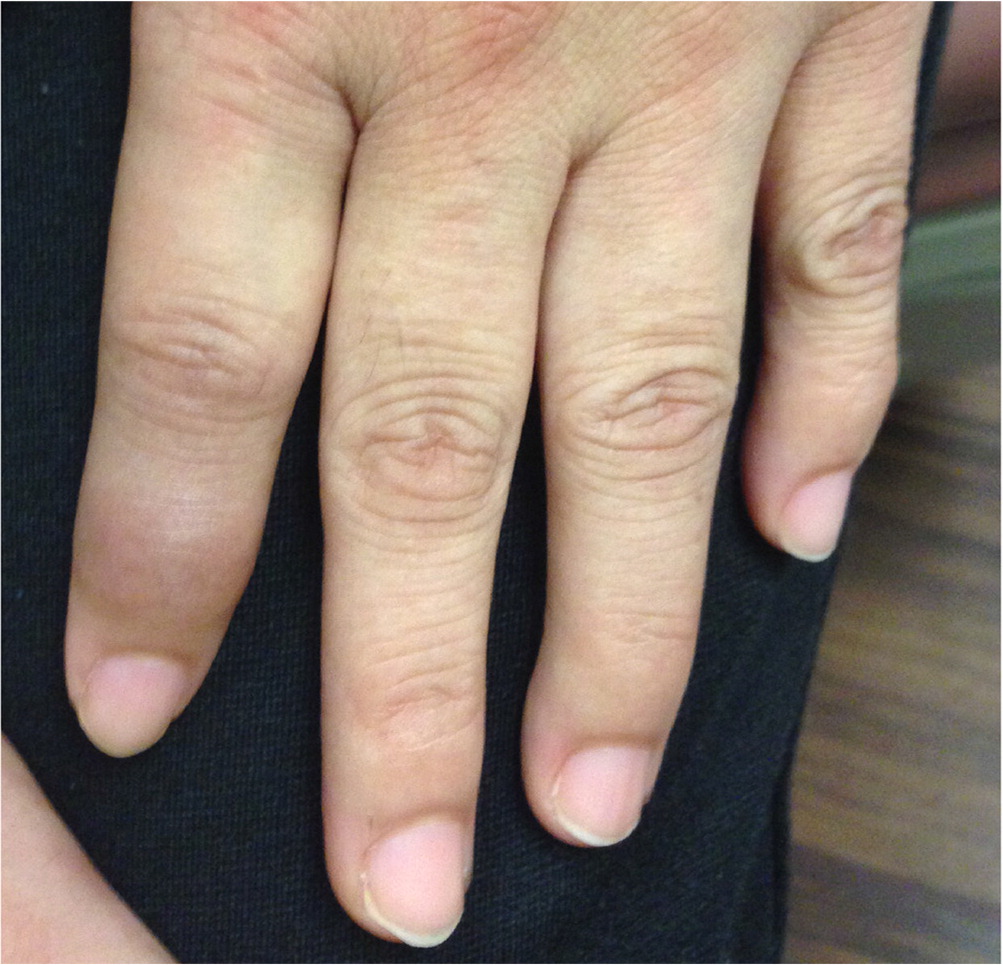
PSORIATIC ARTHRITIS
Comorbidities
Patients with psoriasis have an increased prevalence of metabolic syndrome and obesity, and a propensity to develop diabetes, hypertension, myocardial infarction, and inflammatory bowel disease.12,31–33 All patients with psoriasis should be screened for metabolic syndrome and, if positive, treated to decrease the severity of psoriasis.12 Severe psoriasis is an independent risk factor for atrial fibrillation and thrombosis.34
Psoriasis is also associated with gastrointestinal disorders. In particular, there is a 16% increased risk of colorectal cancer, although guidelines do not recommend earlier or more intensive screening for colorectal cancer in patients with psoriasis.35 Between 4% and 14% of patients with psoriasis will have celiac disease, but removing gluten from their diet does not appear to reduce psoriasis severity.1,36
Depression, suicidal ideation, and anxiety are associated with psoriasis. These conditions can negatively affect quality of life and increase psoriatic severity that may warrant stronger treatment.32
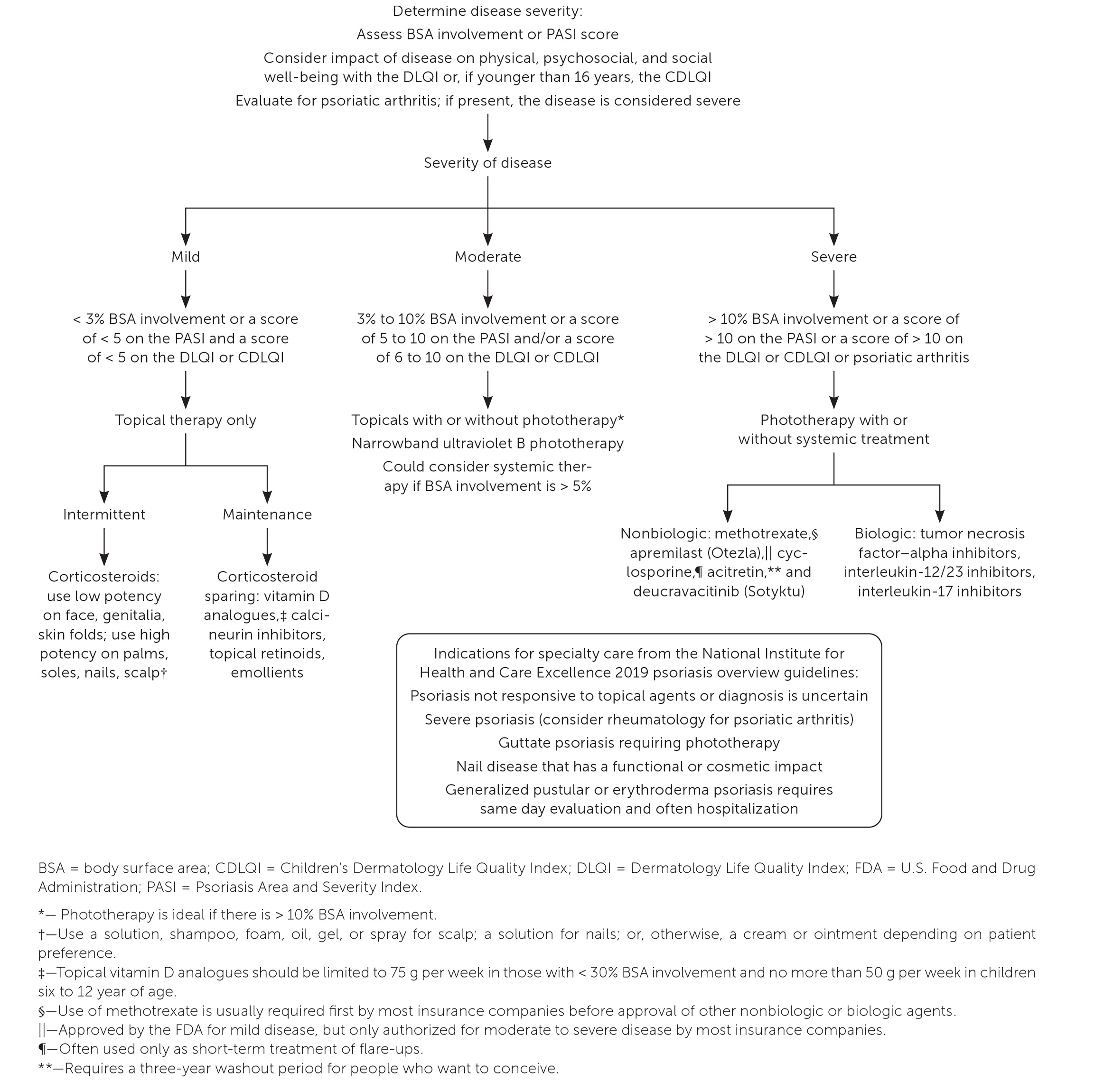
Treatment
Except for erythrodermic psoriasis, which requires prompt initiation of systemic drug therapy and inpatient management of fluids and electrolytes, all other types of psoriasis are treated similarly. Treatment requires regular and consistent use of topical agents, systemic therapy, or phototherapy protocols. Maintaining an ideal body weight, avoiding tobacco products, limiting alcohol intake, and practicing stress reduction techniques are important self-care measures but are not sufficient alone to improve psoriatic severity. Minimizing environmental triggers, such as cold weather, sun exposure, and low humidity, may also be helpful.
| Tool | Link | Indications | Limitations |
|---|---|---|---|
| Psoriasis severity assessment | |||
| Body surface area | https://www.health.state.mn.us/communities/ep/surge/burn/tbsa.html | Used to calculate severity and assess response to treatment; good interrater reliability1 | Can be overestimated |
| Nail Psoriasis Severity Index | https://www.sciencedirect.com/science/article/pii/S0190962203009101?via%3Dihub | Scores each nail for nail bed and nail matrix psoriasis to assess severity42 | Weak consensus for use43 |
| Physician Global Assessment | https://jamanetwork.com/journals/jamadermatology/fullarticle/2039085 | Erythema, induration, and scaling used to measure severity and response to treatment; simple to use1 | Does not consider body surface area |
| Psoriasis Area and Severity Index | https://www.mdcalc.com/calc/10182/psoriasis-area-severity-index-pasi | Erythema, induration, scaling, and affected body surface area used to determine severity and response to treatment1 | Used in research trials and less often in the clinical setting; not accurate for mild psoriasis |
| Psoriasis Symptom Inventory | https://www.tandfonline.com/doi/full/10.3109/09546634.2012.742950 | Patient-reported inventory that measures severity based on itch, redness, scaling, burning, stinging, cracking, flaking, and pain1 | Relies on patient willingness and ability to complete |
| Quality-of-life assessment | |||
| Children’s Dermatology Life Quality Index | https://www.cardiff.ac.uk/medicine/resources/quality-of-life-questionnaires/childrens-dermatology-life-quality-index | Derived from the adult index and available in written and cartoon versions5 | May be difficult to assess pruritus |
| Dermatology Life Quality Index | https://www.cardiff.ac.uk/medicine/resources/quality-of-life-questionnaires/dermatology-life-quality-index | Self-reported assessment based on 10 questions measuring impact of disease on quality of life; assesses severity and response to treatment1 | Relies on patient willingness and ability to complete |
| Psoriatic arthritis assessment | |||
| Classification for Psoriatic Arthritis Criteria | https://www.mdcalc.com/calc/10039/caspar-criteria-psoriatic-arthritis | Classifies patients with inflammatory musculoskeletal disease as having psoriatic arthritis based on a scoring system, and includes skin findings, nail lesions, dactylitis, negative rheumatoid factor, and juxta-articular bone formation on radiography 30 | Used in patients with known inflammatory arthritis |
| Psoriasis Epidemiological Screening Tool | https://www.psoriasis.org/psoriatic-arthritis-screening-test/ | Self-administered to detect psoriatic arthritis | Not validated in children; does not detect axial arthritis or inflammatory back pain40 |
TOPICAL TREATMENTS
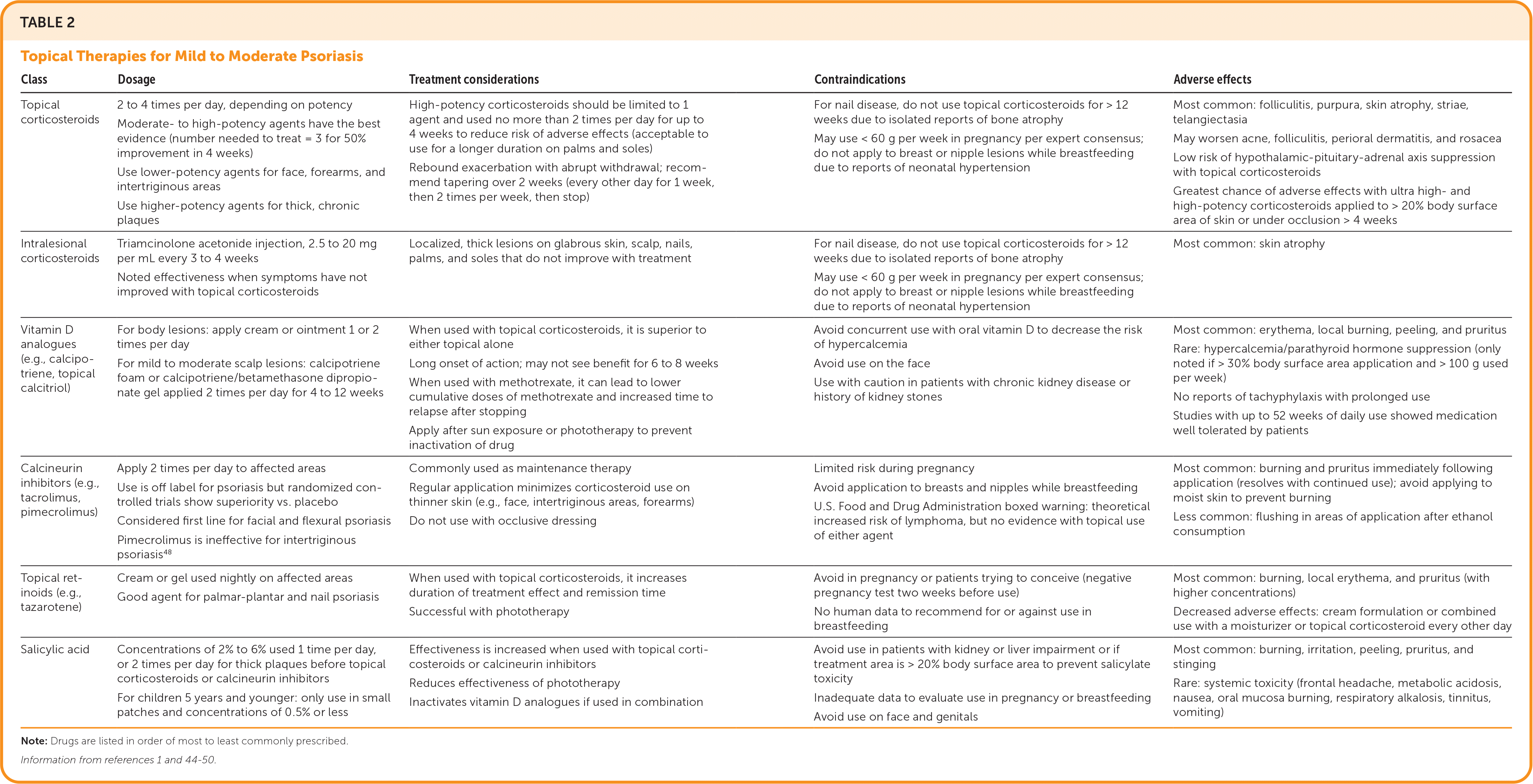
| Class | Dosage | Treatment considerations | Contraindications | Adverse effects |
|---|---|---|---|---|
| Topical corticosteroids | 2 to 4 times per day, depending on potency Moderate- to high-potency agents have the best evidence (number needed to treat = 3 for 50% improvement in 4 weeks) Use lower-potency agents for face, forearms, and intertriginous areas Use higher-potency agents for thick, chronic plaques | High-potency corticosteroids should be limited to 1 agent and used no more than 2 times per day for up to 4 weeks to reduce risk of adverse effects (acceptable to use for a longer duration on palms and soles) Rebound exacerbation with abrupt withdrawal; recommend tapering over 2 weeks (every other day for 1 week, then 2 times per week, then stop) | For nail disease, do not use topical corticosteroids for > 12 weeks due to isolated reports of bone atrophy May use < 60 g per week in pregnancy per expert consensus; do not apply to breast or nipple lesions while breastfeeding due to reports of neonatal hypertension | Most common: folliculitis, purpura, skin atrophy, striae, telangiectasia May worsen acne, folliculitis, perioral dermatitis, and rosacea Low risk of hypothalamic-pituitary-adrenal axis suppression with topical corticosteroids Greatest chance of adverse effects with ultra high- and high-potency corticosteroids applied to > 20% body surface area of skin or under occlusion > 4 weeks |
| Intralesional corticosteroids | Triamcinolone acetonide injection, 2.5 to 20 mg per mL every 3 to 4 weeks Noted effectiveness when symptoms have not improved with topical corticosteroids | Localized, thick lesions on glabrous skin, scalp, nails, palms, and soles that do not improve with treatment | For nail disease, do not use topical corticosteroids for > 12 weeks due to isolated reports of bone atrophy May use < 60 g per week in pregnancy per expert consensus; do not apply to breast or nipple lesions while breastfeeding due to reports of neonatal hypertension | Most common: skin atrophy |
| Vitamin D analogues (e.g., calcipotriene, topical calcitriol) | For body lesions: apply cream or ointment 1 or 2 times per day For mild to moderate scalp lesions: calcipotriene foam or calcipotriene/betamethasone dipropionate gel applied 2 times per day for 4 to 12 weeks | When used with topical corticosteroids, it is superior to either topical alone Long onset of action; may not see benefit for 6 to 8 weeks When used with methotrexate, it can lead to lower cumulative doses of methotrexate and increased time to relapse after stopping Apply after sun exposure or phototherapy to prevent inactivation of drug | Avoid concurrent use with oral vitamin D to decrease the risk of hypercalcemia Avoid use on the face Use with caution in patients with chronic kidney disease or history of kidney stones | Most common: erythema, local burning, peeling, and pruritus Rare: hypercalcemia/parathyroid hormone suppression (only noted if > 30% body surface area application and > 100 g used per week) No reports of tachyphylaxis with prolonged use Studies with up to 52 weeks of daily use showed medication well tolerated by patients |
| Calcineurin inhibitors (e.g., tacrolimus, pimecrolimus) | Apply 2 times per day to affected areas Use is off label for psoriasis but randomized controlled trials show superiority vs. placebo Considered first line for facial and flexural psoriasis Pimecrolimus is ineffective for intertriginous psoriasis48 | Commonly used as maintenance therapy Regular application minimizes corticosteroid use on thinner skin (e.g., face, intertriginous areas, forearms) Do not use with occlusive dressing | Limited risk during pregnancy Avoid application to breasts and nipples while breastfeeding U.S. Food and Drug Administration boxed warning: theoretical increased risk of lymphoma, but no evidence with topical use of either agent | Most common: burning and pruritus immediately following application (resolves with continued use); avoid applying to moist skin to prevent burning Less common: flushing in areas of application after ethanol consumption |
| Topical retinoids (e.g., tazarotene) | Cream or gel used nightly on affected areas Good agent for palmar-plantar and nail psoriasis | When used with topical corticosteroids, it increases duration of treatment effect and remission time Successful with phototherapy | Avoid in pregnancy or patients trying to conceive (negative pregnancy test two weeks before use) No human data to recommend for or against use in breastfeeding | Most common: burning, local erythema, and pruritus (with higher concentrations) Decreased adverse effects: cream formulation or combined use with a moisturizer or topical corticosteroid every other day |
| Salicylic acid | Concentrations of 2% to 6% used 1 time per day, or 2 times per day for thick plaques before topical corticosteroids or calcineurin inhibitors For children 5 years and younger: only use in small patches and concentrations of 0.5% or less | Effectiveness is increased when used with topical corticosteroids or calcineurin inhibitors Reduces effectiveness of phototherapy Inactivates vitamin D analogues if used in combination | Avoid use in patients with kidney or liver impairment or if treatment area is > 20% body surface area to prevent salicylate toxicity Inadequate data to evaluate use in pregnancy or breastfeeding Avoid use on face and genitals | Most common: burning, irritation, peeling, pruritus, and stinging Rare: systemic toxicity (frontal headache, metabolic acidosis, nausea, oral mucosa burning, respiratory alkalosis, tinnitus, vomiting) |
Topical corticosteroids are first-line treatment for all types of psoriatic skin disease and are often combined with vitamin D analogues or topical retinoids to limit the frequency and potency of corticosteroid therapy needed to control disease. In a Cochrane review of 177 randomized controlled trials with 34,808 participants, the combination of a corticosteroid and vitamin D analogue performed better than either agent alone.44 Patients can combine vitamin D and corticosteroids in a 1: 1 mixture daily or apply vitamin D during the week and a higher potency corticosteroid on weekends. Calcipotriene foam and calcipotriene/betamethasone dipropionate gel should be used for four to 12 weeks for the treatment of mild to moderate scalp psoriasis.51–53 Calcineurin inhibitors may be used instead of corticosteroids in areas of thinner skin as maintenance therapy. If plaques improve after four weeks of daily treatment, patients can decrease to twice-weekly treatments to reduce the frequency of flare-ups.54
Creams and ointments are preferred for skin without hair. Ointments tend to be more potent than creams of equal strength. Solutions, shampoos, foams, oils, gels, or sprays are recommended in regions of skin with hair, and selection is based on patient preference.1 Use of emollients may reduce pruritus or burning from topical therapeutic agents and improves outcomes when applied before narrowband ultraviolet B (UVB) phototherapy treatments.5
Topical treatments can be used for nail lesions, but only when no more than two nails are involved. A topical solution of calcipotriene/betamethasone dipropionate and tazarotene results in modest improvement.37 Systemic treatment is more appropriate when multiple nails are affected.
PHOTOTHERAPY
Narrowband UVB phototherapy is effective for plaques and diffuse guttate psoriasis that do not improve with topical treatments. However, phototherapy typically involves multiple visits per week. Insurance often covers in-office treatments, and one six-year study showed that patients spent 40% less in drug costs due to a decreased need for prescription topical medications 12 months after phototherapy.55
SYSTEMIC TREATMENTS
Nonbiologic medications cause global immune suppression and are synthetic in origin. Biologics are derived from living material and are more selective in how they disrupt the immune system to prevent immune-mediated disorders. Although systemic treatments are generally reserved for moderate to severe psoriasis, one systemic nonbiologic medication (apremilast [Otezla]) has been approved by the U.S. Food and Drug Administration for mild disease.56
Although individual guidelines vary in their definitions of psoriasis severity, systemic agents can generally be considered when the involved BSA exceeds 5%.38,57,58 BSA can be estimated using the surface of the hand (from the wrist to the fingers and thumb closed together), representing 1% BSA.1 Dosages, contraindications, and adverse effects of various systemic agents are outlined in eTable A. Recommendations for monitoring during therapy are listed in eTable B.
| Medication | Dosage | Treatment considerations | Adverse effects |
|---|---|---|---|
| Nonbiologics | |||
| Methotrexate (FDA approved in 1972) | Starting dose: 7.5 mg; therapeutic dose: 15 mg; maximum dose: 30 mg once per week orally, subcutaneously, intramuscularly, or intravenously May give divided doses orally twice per day 3 times per week (for decreased gastrointestinal adverse effects) Start with 2.5- to 5-mg doses for those with possible drug interactions, kidney disease, or diabetes mellitus | 1 mg of folic acid supplementation is recommended once per day except on days when taking methotrexate (decreases gastrointestinal and liver abnormalities) Cost is significantly less than tumor necrosis factor-alpha inhibitors Avoid in alcohol use disorder and liver disease Avoid if any known bone marrow suppression or immunodeficiency syndromes Avoid if abnormal baseline kidney and liver function | Common: anorexia, fatigue, increased risk of infection (including reactivation of latent tuberculosis, hepatitis B and C, or Epstein-Barr virus), liver enzyme elevation, nausea, stomatitis Rare: B cell lymphoma, cirrhosis, hemorrhagic enteritis, nephrotoxicity, pancytopenia, pneumonitis, Steven-Johnson syndrome/toxic epidermal necrolysis |
| Apremilast (Otezla; FDA approved in 2014) | 30 mg orally twice per day Initial dose: 10 mg once per day, titrated up by 10 mg per day over first 5 days If glomerular filtration rate is < 30 mL per minute per 1.73 m2, decrease to 30 mg once per day to prevent adverse effects or toxicity | Effective for scalp, palmar, and plantar involvement and psoriatic arthritis No strong evidence for concurrent use of systemic treatments or phototherapy Consider discontinuation if > 5% weight loss Avoid concurrent use of cytochrome P450 inducers, which will decrease effectiveness | Common: diarrhea, nasopharyngitis, nausea, tension headache; upper respiratory tract infection, 70% to 80% of gastrointestinal adverse effects occurred within the first 2 weeks and 60% to 65% resolved after the first month Rare: angioedema, depression, suicidal ideation |
| Cyclosporine (FDA approved in 1997) | 2.5 to 4 mg per kg orally, in divided doses twice per day, for 12 to 16 weeks Start 2.5 mg per kg per day and increase by 0.5 mg per kg per day every 2 weeks to a maximum dose of 4 mg per kg per day Discontinue if inadequate improvement at maximum dose for 6 weeks | Rapid control of severe, recalcitrant disease, acute flare-ups, and erythroderma; can be used for bridge therapy until safer long-term treatment can be found Relapse common after discontinuation Avoid phototherapy when taking cyclosporine because it increases the risk of photocarcinogenesis (sequential use is effective and safer) Do not use with statins, thiazide and potassium sparing diuretics, warfarin, or nonsteroidal anti-inflammatory drugs due to increased risk of adverse effects Not used as long-term treatment due to multiple serious adverse effects | Common: headache, hypertension (if hypertension develops, decrease dose; if it does not normalize, stop medication), musculoskeletal pain, nephrotoxicity (19% to 24% will develop reversible impairment with short-term treatment), paresthesia Rare: kidney fibrosis and irreversible nephrotoxicity (increased risk if treatment duration is > 2 years) |
| Acitretin (FDA approved in 1997) | 25 to 50 mg orally once per day with food Can take 36 months for full treatment response | Combined with phototherapy, it is more effective than monotherapy Not immunosuppressive, can be used in those with HIV Avoid in severe kidney or liver disease | Common: brittle nails, cheilitis, dry eye, epistaxis, hair loss (at doses > 17.5 mg per day in women), hyperlipidemia (25% to 50%), itching/burning skin, xerosis Rare: capillary leak syndrome, exfoliative dermatitis, hepato-toxicity, hypervitaminosis A syndrome, pseudotumor cerebri, thromboembolism |
| Deucravacitinib (Sotyktu; FDA approved for adults only in 2022) | 6 mg orally once per day | Screen for and treat tuberculosis before starting If liver function test result is three times the upper limit of normal, evaluate for drug-induced liver injury Not recommended for patients with active hepatitis B or C | Common: infection, liver enzyme and triglyceride elevation Rare: increased risk of lymphoma, rhabdomyolysis |
| Biologics | |||
| Adalimumab (FDA approved in 2005 for adults with psoriatic arthritis and in 2008 for severe plaque psoriasis) | 80 mg subcutaneously on day 1, then 40 mg on day 8, and continue 40 mg once every 2 weeks for maintenance | Used with acitretin and methotrexate (5 to 15 mg per week), it is safe and increases long-term effectiveness Do not use in patients with active infection, history of lymphoreticular malignancy, New York Heart Association class III or IV congestive heart failure, or untreated hepatitis B infection | FDA boxed warning: serious infection risk (i.e., opportunistic infections, including tuberculosis and invasive fungal infections) and malignancy risk (i.e., lymphoma and skin cancer) Common: diarrhea, fever, injection site reaction, pruritus, rash, upper respiratory tract infection, urticaria Rare: exacerbation or new onset of congestive heart failure, drug-induced reversible lupus, or interstitial lung disease |
| Etanercept (FDA approved in 2004 for adults and children older than 4 years with moderate to severe plaque, scalp, or nail psoriasis, or psoriatic arthritis) | 50 mg subcutaneously once per week or 25 mg subcutaneously twice per week (72 to 96 hours apart) For severe chronic plaque psoriasis, start 50 mg subcutaneously twice per week for 3 months, then decrease to 50 mg once per week Patients 4 to 17 years of age: 0.8 mg per kg per dose once per week for < 63 kg and 50 mg once per week if ≥ 63 kg | Can be used with topical agents, methotrexate, and acitretin (decreased risk of cutaneous squamous cell carcinoma when used with acitretin) Do not use in patients with untreated hepatitis B infection, history of lymphoreticular malignancy, active infection, or New York Heart Association class III or IV congestive heart failure | FDA boxed warning: serious infection risk (i.e., opportunistic infections, including tuberculosis and invasive fungal infections) and malignancy risk (i.e., lymphoma and skin cancer) Common: diarrhea, fever, injection site reaction, pruritus, rash, upper respiratory tract infection, urticaria Rare: exacerbation or new onset of congestive heart failure, drug-induced reversible lupus, or interstitial lung disease |
| Infliximab (FDA approved in 2005 for adults with psoriatic arthritis and in 2006 for severe plaque psoriasis) | 5 mg per kg per dose intravenously once every 8 weeks (initially, and if there is a break in treatment, 5 mg per kg per dose on weeks 0, 2, and 6) Increase in the risk of infusion reactions and loss of effectiveness if > 8 weeks between infusions due to antibodies to infliximab | Can be used with topicals, acitretin, or methotrexate for synergistic effect Do not use in patients with active infection, history of lymphoreticular malignancy, New York Heart Association class III or IV congestive heart failure, or untreated hepatitis B infection | FDA boxed warning: serious infection risk (i.e., opportunistic infections, including tuberculosis and invasive fungal infections) and malignancy risk (i.e., lymphoma and skin cancer) Common: diarrhea, fever, injection site reaction, pruritus, rash, upper respiratory tract infection, urticaria Rare: exacerbation or new onset of congestive heart failure, drug-induced reversible lupus, or interstitial lung disease |
| Ixekizumab (Taltz; FDA approved in 2016 for plaque psoriasis, 2017 for psoriatic arthritis, 2020 for pediatric plaque psoriasis) | Plaque psoriasis Adults: 160 mg subcutaneously the first week, then 80 mg subcutaneously every 2 weeks through week 12, then 80 mg every 4 weeks Children (6 to 17 years of age): < 25 kg: 20 mg subcutaneously every 4 weeks, then start 40 mg subcutaneously once per week 25 to 50 kg: 40 mg subcutaneously every 4 weeks, then start 80 mg subcutaneously once per week > 50 kg: 80 mg subcutaneously every 4 weeks, then start 160 mg subcutaneously once per week Psoriatic arthritis Adults: 80 mg subcutaneously every 4 weeks after starting dose of 160 mg subcutaneously the first week | Avoid in patients with history of or active inflammatory bowel disease or tuberculosis infection | Common: injection site reaction, nausea, neutropenia, thrombocytopenia, tinea infections Rare: hypersensitivity reactions (e.g., anaphylaxis, angioedema), inflammatory bowel disease |
| Biologics | |||
| Secukinumab (Cosentyx; FDA approved in 2015 for adults with plaque psoriasis, in 2021 expanded to children and adults with plaque psoriasis, nail psoriasis, and psoriatic arthritis) | Plaque psoriasis/nail psoriasis Adults: 300 mg subcutaneously once every 4 weeks (loading dose of 300 mg once per week for 5 weeks) Children (6 to 17 years of age): < 50 kg: 75 mg* ≥ 50 kg: 150 mg* Psoriatic arthritis Adults: 150 mg subcutaneously once every 4 weeks (loading dose: 150 mg once per week for 5 weeks) Children (2 to 17 years of age): 15 to 50 kg: 75 mg* > 50 kg: 150 mg* | Avoid in patients with history of or active inflammatory bowel disease or tuberculosis infection Shown to be superior to phototherapy in patients with new-onset, moderate to severe plaque psoriasis; the high and sustained skin clearance observed indicates that biologic treatment for psoriasis may be more effective if used early in the disease course | Common: diarrhea, infection, nasopharyngitis, neutropenia, upper respiratory tract infection Rare: hypersensitivity reactions (e.g., anaphylaxis, angioedema), inflammatory bowel disease, mucocutaneous Candida infections, sepsis, toxic shock syndrome |
| Ustekinumab (Stelara; FDA approved for psoriatic arthritis and plaque psoriasis in adults and children 6 to 17 years of age) | Adults < 100 kg: 45 mg subcutaneously every 12 weeks (45 mg subcutaneously on weeks 0, 4, and 12 to start) Adults ≥ 100 kg: 90 mg subcutaneously every 12 weeks (90 mg subcutaneously on weeks 0, 4, and 12 to start) Children 6 to 17 years of age < 60 kg: 0.75 mg per kg per dose subcutaneously every 12 weeks (0.75 mg per kg per dose on week 0, 4, 12 to start) Children 6 to 17 years of age ≥ 60 kg: 45 mg subcutaneously at 0 and 4 weeks, then 45 mg subcutaneously every 12 weeks | Can be used with acitretin, methotrexate, apremilast, and cyclosporine for synergistic effects Using with methotrexate leads to longer drug survival in biologic-naive patients Avoid in patients with untreated hepatitis B or history of lymphoreticular malignancy, active infection, or | Common: abdominal pain, arthralgias, back pain, diarrhea, fatigue, headache, infection, injection site reaction, mouth or throat pain, pruritus Rare: hypersensitivity reactions (e.g., anaphylaxis, angioedema), malignancy, pneumonia (i.e., interstitial, eosinophilic, or cryptogenic organizing), posterior reversible encephalopathy syndrome |
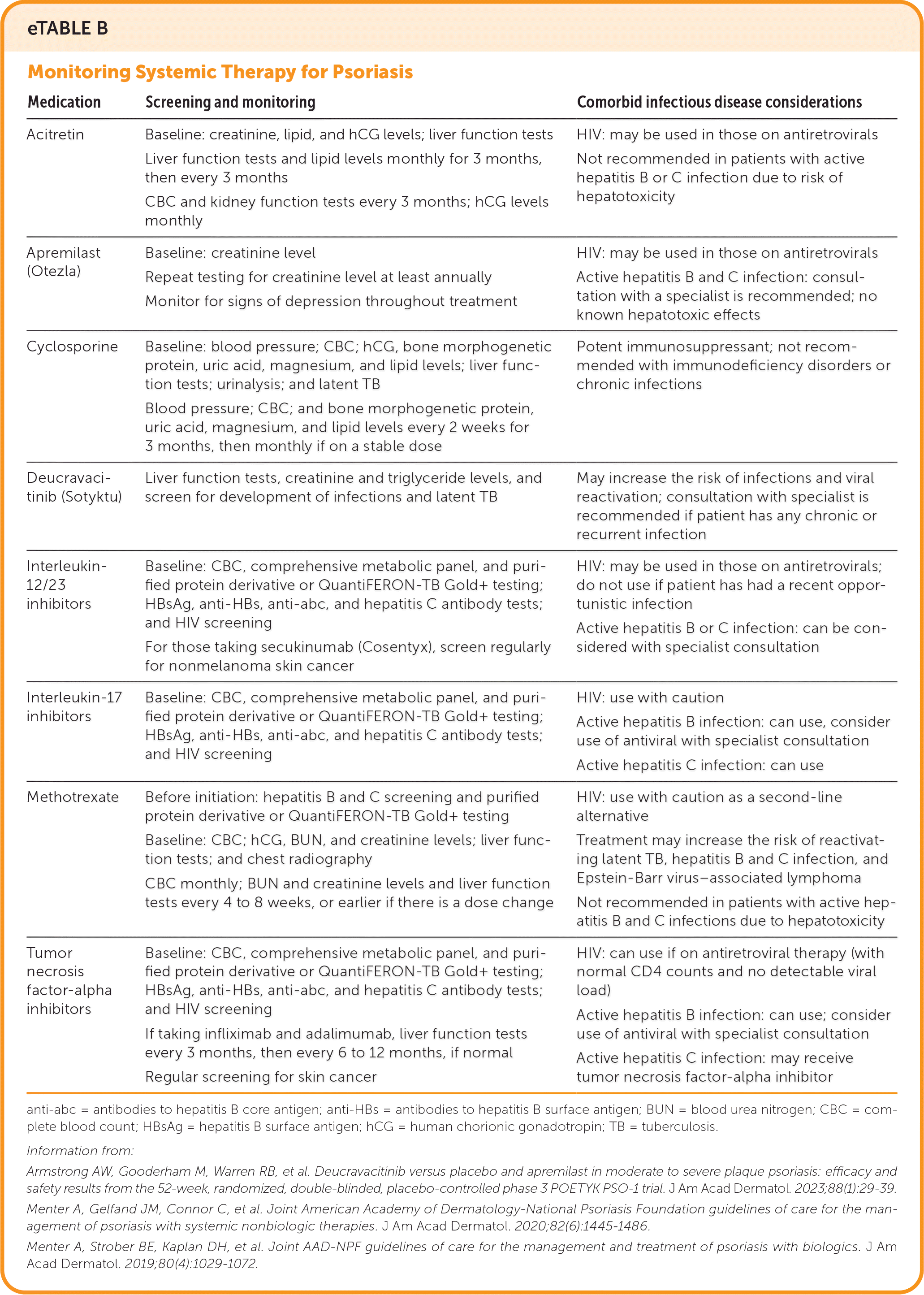
| Medication | Screening and monitoring | Comorbid infectious disease considerations |
|---|---|---|
| Acitretin | Baseline: creatinine, lipid, and hCG levels; liver function tests Liver function tests and lipid levels monthly for 3 months, then every 3 months CBC and kidney function tests every 3 months; hCG levels monthly | HIV: may be used in those on antiretrovirals Not recommended in patients with active hepatitis B or C infection due to risk of hepatotoxicity |
| Apremilast (Otezla) | Baseline: creatinine level Repeat testing for creatinine level at least annually Monitor for signs of depression throughout treatment | HIV: may be used in those on antiretrovirals Active hepatitis B and C infection: consultation with a specialist is recommended; no known hepatotoxic effects |
| Cyclosporine | Baseline: blood pressure; CBC; hCG, bone morphogenetic protein, uric acid, magnesium, and lipid levels; liver function tests; urinalysis; and latent TB Blood pressure; CBC; and bone morphogenetic protein, uric acid, magnesium, and lipid levels every 2 weeks for 3 months, then monthly if on a stable dose | Potent immunosuppressant; not recommended with immunodeficiency disorders or chronic infections |
| Deucravacitinib (Sotyktu) | Liver function tests, creatinine and triglyceride levels, and screen for development of infections and latent TB | May increase the risk of infections and viral reactivation; consultation with specialist is recommended if patient has any chronic or recurrent infection |
| Interleukin- 12/23 inhibitors | Baseline: CBC, comprehensive metabolic panel, and purified protein derivative or QuantiFERON-TB Gold+ testing; HBsAg, anti-HBs, anti-abc, and hepatitis C antibody tests; and HIV screening For those taking secukinumab (Cosentyx), screen regularly for nonmelanoma skin cancer | HIV: may be used in those on antiretrovirals; do not use if patient has had a recent opportunistic infection Active hepatitis B or C infection: can be considered with specialist consultation |
| Interleukin-17 inhibitors | Baseline: CBC, comprehensive metabolic panel, and purified protein derivative or QuantiFERON-TB Gold+ testing; HBsAg, anti-HBs, anti-abc, and hepatitis C antibody tests; and HIV screening | HIV: use with caution Active hepatitis B infection: can use, consider use of antiviral with specialist consultation Active hepatitis C infection: can use |
| Methotrexate | Before initiation: hepatitis B and C screening and purified protein derivative or QuantiFERON-TB Gold+ testing Baseline: CBC; hCG, BUN, and creatinine levels; liver function tests; and chest radiography CBC monthly; BUN and creatinine levels and liver function tests every 4 to 8 weeks, or earlier if there is a dose change | HIV: use with caution as a second-line alternative Treatment may increase the risk of reactivating latent TB, hepatitis B and C infection, and Epstein-Barr virus–associated lymphoma Not recommended in patients with active hepatitis B and C infections due to hepatotoxicity |
| Tumor necrosis factor-alpha inhibitors | Baseline: CBC, comprehensive metabolic panel, and purified protein derivative or QuantiFERON-TB Gold+ testing; HBsAg, anti-HBs, anti-abc, and hepatitis C antibody tests; and HIV screening If taking infliximab and adalimumab, liver function tests every 3 months, then every 6 to 12 months, if normal Regular screening for skin cancer | HIV: can use if on antiretroviral therapy (with normal CD4 counts and no detectable viral load) Active hepatitis B infection: can use; consider use of antiviral with specialist consultation Active hepatitis C infection: may receive tumor necrosis factor-alpha inhibitor |
If there is no contraindication to systemic treatment, the choice of specific agent is individualized based on patient and physician preference and insurance authorization. Adalimumab and infliximab are more effective than methotrexate for cutaneous psoriasis; however, most insurance requires failure of methotrexate before approving these medications.57
If methotrexate is used as initial systemic treatment and the patient does not have a 25% reduction in Psoriasis Area and Severity Index score after four weeks of treatment, switching to another systemic treatment is recommended.59 An alternative to methotrexate alone is combining biologic treatments with methotrexate to decrease formation of anti-drug antibodies and improve effectiveness over time.
Patients using systemic agents must remain current on routine vaccinations and cancer screenings. Consultation with allergy and immunology is recommended before administration of any live vaccines. Live vaccines should not be given to patients taking biologic therapies; however, biologic therapy can be restarted one to two weeks following a live vaccine.38 Patients taking tumor necrosis factor-alpha inhibitors require annual skin examinations to assess for nonmelanoma skin cancers.38
Temporary Discontinuation of Therapy
Primary care physicians should be aware of situations in which systemic treatments should be temporarily discontinued. For example, ustekinumab (Stelara) should be temporarily discontinued for febrile illnesses requiring antibiotic treatment. It can be restarted after full resolution of symptoms and completion of antibiotics. Tumor necrosis factor-alpha inhibitors (i.e., etanercept or infliximab) do not need to be stopped for uncomplicated infections requiring antibiotics.
For procedures with a low risk of postoperative infection (e.g., hernia repair, endoscopy, or arthroscopy), all biologics may be continued. For procedures with a moderate risk of postoperative infection (e.g., intra-abdominal surgery without bowel resection, intrathoracic surgery without lung resection, gynecologic surgery) or high risk of infection (e.g., joint replacement, oncologic surgery, bowel resection), systemic therapies usually require cessation and should be discussed with the surgical team.38 If biologic agents should be discontinued, therapy should be stopped three to four half-lives before surgery.38 Infliximab can be restarted three to four weeks after surgery, etanercept 12 days after surgery, and adalimumab 56 days after moderate- to high-risk elective surgeries if the wound is fully healed and there are no postoperative complications.60
Special Considerations for Psoriasis in Children and Adolescents
Plaque psoriasis in children usually presents in adolescents; however, 28% of children with guttate psoriasis develop the condition before adolescence. In children who develop gut-tate psoriasis after a positive streptococcal test, 60% will have complete remission within months. Children with no active streptococcal infection at the time of guttate psoriasis diagnosis have an increased risk of progressing to chronic plaque psoriasis.61
Only 0.7% to 1.2% of children will develop psoriatic arthritis, although it represents 6% to 8% of childhood inflammatory arthritis cases.5,61 Oligoarticular symptoms, dactylitis, and a family history of psoriasis in younger children increase the likelihood of psoriatic arthritis, whereas enthesitis (inflammation of the insertion sites of tendons and ligaments) and axial joint involvement are more common in older children and teenagers with psoriatic arthritis.5
Treatment is based on BSA and disease impact. All corticosteroid potencies can be used and applied once or twice daily for up to 14 days, but low- to moderate-potency corticosteroids are preferred for children younger than six years.
Calcipotriene/betamethasone dipropionate ointment that is applied daily for up to four weeks is safe and effective for children older than 12 years, and treatment can be extended up to eight weeks on the scalp.5 To limit the risk of hypercalcemia, vitamin D analogues should be limited to 75 g per week in those with less than 30% BSA involvement, and no more than 50 g per week in children six to 12 years of age.
Narrowband UVB phototherapy is safe and effective for children with 10% to 25% BSA involvement of any form of cutaneous psoriasis.61 In a retrospective study, after 12 days of coal tar use before narrowband UVB phototherapy, 64% had clearance of lesions and 43% had sustained remission for one year.5
Nonbiologic systemic agents should be used only in children with moderate to severe psoriasis that does not improve with topical therapy or narrowband UVB phototherapy.5 Methotrexate is the most widely used agent due to long-term effectiveness and safety data, followed by biologics, such as etanercept in those four years and older, and ustekinumab, ixekizumab (Taltz), and secukinumab (Cosentyx) in children older than six years. Adalimumab is not approved by the U.S. Food and Drug Administration for use in children.
Physicians should have a high index of suspicion for childhood and adolescent depression and substance use due to bullying and shaming.
Fertility, Pregnancy, and Lactation Considerations
During pregnancy, about two-thirds of women with psoriasis will have improvement, and fewer than one-third will have increased severity.39 Topicals may be used by patients trying to conceive and during pregnancy because of their minimal systemic absorption. However, calcineurin inhibitors should be used only on less than 1% BSA, and retinoids should not be used. Topical corticosteroids should be limited to less than 60 g per week during pregnancy, although a recent Cochrane review found no causal association between maternal use of any potency topical corticosteroid and congenital abnormality.1 While breastfeeding, topicals must not be applied to the breast or skin that comes in contact with the infant.
Narrowband UVB phototherapy is safe in men and women who are trying to conceive and pregnant and breastfeeding patients. However, there is a dose-dependent reduction in serum folate during narrowband UVB phototherapy. All women of childbearing age should take at least 0.8 mg of folate daily to ensure adequate supplementation.62
Methotrexate requires a three-month washout period before trying to conceive, and it should be avoided in patients who are pregnant or breastfeeding. Although there are no human studies, apremilast was associated with teratogenicity and pregnancy loss in animals. eTable C summarizes the safety of nonbiologic and biologic medications in patients with psoriasis who are pregnant, breastfeeding, or trying to conceive.
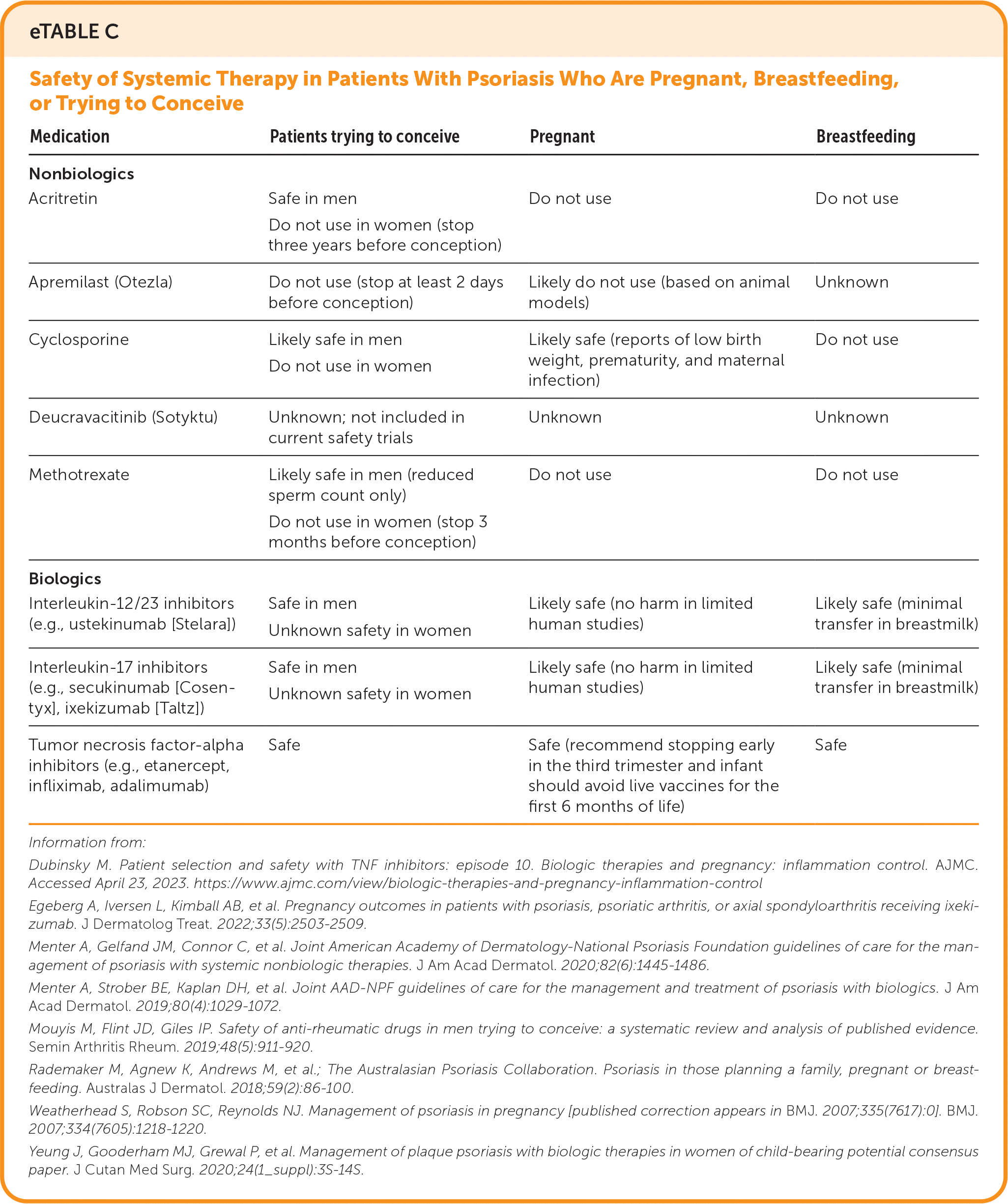
| Medication | Patients trying to conceive | Pregnant | Breastfeeding |
|---|---|---|---|
| Nonbiologics | |||
| Acritretin | Safe in men Do not use in women (stop three years before conception) | Do not use | Do not use |
| Apremilast (Otezla) | Do not use (stop at least 2 days before conception) | Likely do not use (based on animal models) | Unknown |
| Cyclosporine | Likely safe in men Do not use in women | Likely safe (reports of low birth weight, prematurity, and maternal infection) | Do not use |
| Deucravacitinib (Sotyktu) | Unknown; not included in current safety trials | Unknown | Unknown |
| Methotrexate | Likely safe in men (reduced sperm count only) Do not use in women (stop 3 months before conception) | Do not use | Do not use |
| Biologics | |||
| Interleukin-12/23 inhibitors (e.g., ustekinumab [Stelara]) | Safe in men Unknown safety in women | Likely safe (no harm in limited human studies) | Likely safe (minimal transfer in breastmilk) |
| Interleukin-17 inhibitors (e.g., secukinumab [Cosentyx], ixekizumab [Taltz]) | Safe in men Unknown safety in women | Likely safe (no harm in limited human studies) | Likely safe (minimal transfer in breastmilk) |
| Tumor necrosis factor-alpha inhibitors (e.g., etanercept, infliximab, adalimumab) | Safe | Safe (recommend stopping early in the third trimester and infant should avoid live vaccines for the first 6 months of life) | Safe |
This article updates previous articles on this topic by Weigle and McBane63; Luba and Stulberg64; Pardasani, et al.65; and Federman, et al.66
Data Sources: A PubMed search was performed in the Clinical Queries function using the key terms psoriasis, plaque psoriasis, guttate psoriasis, and pustular psoriasis. The search was limited to publications from 2012 or later. Meta-analyses, systematic reviews, randomized controlled trials, clinical trials, NICE guidelines, Essential Evidence Plus, and Choosing Wisely recommendations were included. Search dates: September through October 2022, March through April 2023, and September 16, 2023.
The authors thank Matt Perez, MD, and Jane Kerford, MD, for assistance with acquiring photos for the manuscript.
The contents of this article are solely the views of the authors and do not necessarily represent the official views of the U.S. Air Force, U.S. military at large, U.S. Department of Defense, or U.S. government.
